Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-02-11 , DOI: 10.1016/j.comptc.2020.112747 Tianlei Zhang , Kaiyue Zhai , Yongqi Zhang , Lin Geng , Zerong Geng , Mi Zhou , Yousong Lu , Xianzhao Shao , Makroni Lily
|
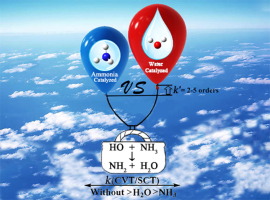
A comprehensive investigation of the roles of H2O and NH3 on the HO + NH3 → NH2 + H2O reaction in the troposphere has been carried out by CCSD(T)-F12a/cc-pVDZ-F12//M06-2X/6-311+G(2d,2p) method, and the canonical variational transition state theory with small curvature tunneling correction. The results show that both H2O and NH3 catalyzed reactions prefer the single hydrogen atom transfers (HAT) pathways than the double HAT routes. Meanwhile, the catalytic effect of H2O is more obvious as compared with NH3 with its effective rate constant (k'(WM)) larger by 2-5 orders of magnitude. However, within the temperature range of 213-320 K, the calculated value of k'(WM) is smaller by 5-9 orders of magnitude than the corresponding rate constant of the naked reaction. This result is in agreement with H2O catalyzed OH + CH2CH2, OH + CH2O and OH + CH2NH reactions, where water-assisted reaction cannot accelerate the reaction.
中文翻译:

水和氨对对流层中HO + NH 3 →NH 2 + H 2 O反应的影响:单氢和双氢原子转移途径之间的竞争
CCSD(T)-F12a / cc-pVDZ-F12 // M06对H 2 O和NH 3在对流层中HO + NH 3 →NH 2 + H 2 O反应中的作用进行了全面研究-2X / 6-311 + G(2 d,2 p)方法,以及具有小曲率隧穿校正的规范变分过渡态理论。结果表明,H 2 O和NH 3催化的反应均比单HAT途径更倾向于单氢原子转移(HAT)途径。同时,H 2 O的催化速率比NH 3更为明显,其有效速率常数(k' (WM) )数量级的2-5订单大。但是,在213-320 K的温度范围内,k ' (WM)的计算值比裸反应的相应速率常数小5-9个数量级。该结果与H 2 O催化的OH + CH 2 CH 2,OH + CH 2 O和OH + CH 2 NH反应一致,其中水辅助反应不能促进反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号