当前位置:
X-MOL 学术
›
Pharmacogenomics J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data.
The Pharmacogenomics Journal ( IF 2.9 ) Pub Date : 2020-02-11 , DOI: 10.1038/s41397-020-0162-5 Nita A Limdi 1 , Larisa H Cavallari 2 , Craig R Lee 3 , William B Hillegass 4 , Ann M Holmes 5 , Todd C Skaar 6 , Maria Pisu 7 , Chrisly Dillon 1 , Amber L Beitelshees 8 , Philip E Empey 9 , Julio D Duarte 2 , Vakaramoko Diaby 10 , Yan Gong 2 , Julie A Johnson 2 , John Graves 11 , Shawn Garbett 11 , Zilu Zhou 11 , Josh F Peterson 11 ,
The Pharmacogenomics Journal ( IF 2.9 ) Pub Date : 2020-02-11 , DOI: 10.1038/s41397-020-0162-5 Nita A Limdi 1 , Larisa H Cavallari 2 , Craig R Lee 3 , William B Hillegass 4 , Ann M Holmes 5 , Todd C Skaar 6 , Maria Pisu 7 , Chrisly Dillon 1 , Amber L Beitelshees 8 , Philip E Empey 9 , Julio D Duarte 2 , Vakaramoko Diaby 10 , Yan Gong 2 , Julie A Johnson 2 , John Graves 11 , Shawn Garbett 11 , Zilu Zhou 11 , Josh F Peterson 11 ,
Affiliation
|
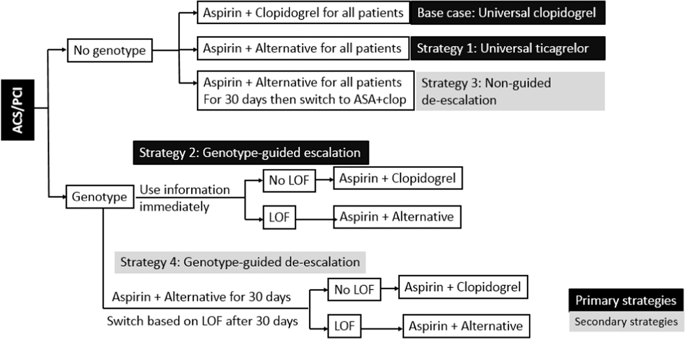
Current guidelines recommend dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 inhibitors following percutaneous coronary intervention (PCI). CYP2C19 genotype can guide DAPT selection, prescribing ticagrelor or prasugrel for loss-of-function (LOF) allele carriers (genotype-guided escalation). Cost-effectiveness analyses (CEA) are traditionally grounded in clinical trial data. We conduct a CEA using real-world data using a 1-year decision-analytic model comparing primary strategies: universal empiric clopidogrel (base case), universal ticagrelor, and genotype-guided escalation. We also explore secondary strategies commonly implemented in practice, wherein all patients are prescribed ticagrelor for 30 days post PCI. After 30 days, all patients are switched to clopidogrel irrespective of genotype (nonguided de-escalation) or to clopidogrel only if patients do not harbor an LOF allele (genotype-guided de-escalation). Compared with universal clopidogrel, both universal ticagrelor and genotype-guided escalation were superior with improvement in quality-adjusted life years (QALY's). Only genotype-guided escalation was cost-effective ($42,365/QALY) and demonstrated the highest probability of being cost-effective across conventional willingness-to-pay thresholds. In the secondary analysis, compared with the nonguided de-escalation strategy, although genotype-guided de-escalation and universal ticagrelor were more effective, with ICER of $188,680/QALY and $678,215/QALY, respectively, they were not cost-effective. CYP2C19 genotype-guided antiplatelet prescribing is cost-effective compared with either universal clopidogrel or universal ticagrelor using real-world implementation data. The secondary analysis suggests genotype-guided and nonguided de-escalation may be viable strategies, needing further evaluation.
中文翻译:

CYP2C19 指导的抗血小板治疗对急性冠脉综合征和经皮冠状动脉介入治疗患者的成本效益,由真实世界数据提供。
目前的指南建议在经皮冠状动脉介入治疗 (PCI) 后使用由阿司匹林和一种 P2Y12 抑制剂组成的双重抗血小板治疗 (DAPT)。CYP2C19 基因型可以指导 DAPT 选择,为功能丧失 (LOF) 等位基因携带者开具替格瑞洛或普拉格雷(基因型指导的升级)。成本效益分析 (CEA) 传统上基于临床试验数据。我们使用真实世界数据进行 CEA,使用 1 年决策分析模型比较主要策略:通用经验性氯吡格雷(基本案例)、通用替格瑞洛和基因型指导的升级。我们还探讨了实践中通常实施的次要策略,其中所有患者在 PCI 后 30 天都服用替格瑞洛。30天后,不论基因型如何,所有患者都改用氯吡格雷(非指导性降阶梯),或者只有在患者没有 LOF 等位基因时才换用氯吡格雷(基因型指导降阶梯)。与通用氯吡格雷相比,通用替格瑞洛和基因型指导的升级都在质量调整生命年(QALY's)方面具有优势。只有基因型指导的升级具有成本效益(42,365 美元/QALY),并且在传统的支付意愿阈值中表现出最高的成本效益。在二次分析中,与非引导降阶梯策略相比,虽然基因型引导降阶梯和通用替格瑞洛更有效,ICER 分别为 188,680 美元/QALY 和 678,215 美元/QALY,但它们并不具有成本效益。与使用真实世界实施数据的通用氯吡格雷或通用替格瑞洛相比,CYP2C19 基因型指导的抗血小板处方具有成本效益。二次分析表明,基因型引导和非引导降级可能是可行的策略,需要进一步评估。
更新日期:2020-02-11
中文翻译:

CYP2C19 指导的抗血小板治疗对急性冠脉综合征和经皮冠状动脉介入治疗患者的成本效益,由真实世界数据提供。
目前的指南建议在经皮冠状动脉介入治疗 (PCI) 后使用由阿司匹林和一种 P2Y12 抑制剂组成的双重抗血小板治疗 (DAPT)。CYP2C19 基因型可以指导 DAPT 选择,为功能丧失 (LOF) 等位基因携带者开具替格瑞洛或普拉格雷(基因型指导的升级)。成本效益分析 (CEA) 传统上基于临床试验数据。我们使用真实世界数据进行 CEA,使用 1 年决策分析模型比较主要策略:通用经验性氯吡格雷(基本案例)、通用替格瑞洛和基因型指导的升级。我们还探讨了实践中通常实施的次要策略,其中所有患者在 PCI 后 30 天都服用替格瑞洛。30天后,不论基因型如何,所有患者都改用氯吡格雷(非指导性降阶梯),或者只有在患者没有 LOF 等位基因时才换用氯吡格雷(基因型指导降阶梯)。与通用氯吡格雷相比,通用替格瑞洛和基因型指导的升级都在质量调整生命年(QALY's)方面具有优势。只有基因型指导的升级具有成本效益(42,365 美元/QALY),并且在传统的支付意愿阈值中表现出最高的成本效益。在二次分析中,与非引导降阶梯策略相比,虽然基因型引导降阶梯和通用替格瑞洛更有效,ICER 分别为 188,680 美元/QALY 和 678,215 美元/QALY,但它们并不具有成本效益。与使用真实世界实施数据的通用氯吡格雷或通用替格瑞洛相比,CYP2C19 基因型指导的抗血小板处方具有成本效益。二次分析表明,基因型引导和非引导降级可能是可行的策略,需要进一步评估。











































 京公网安备 11010802027423号
京公网安备 11010802027423号