当前位置:
X-MOL 学术
›
Cell. Mol. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural understanding of T cell receptor triggering.
Cellular & Molecular Immunology ( IF 21.8 ) Pub Date : 2020-02-11 , DOI: 10.1038/s41423-020-0367-1 Xinyi Xu 1 , Hua Li 1 , Chenqi Xu 1, 2
Cellular & Molecular Immunology ( IF 21.8 ) Pub Date : 2020-02-11 , DOI: 10.1038/s41423-020-0367-1 Xinyi Xu 1 , Hua Li 1 , Chenqi Xu 1, 2
Affiliation
|
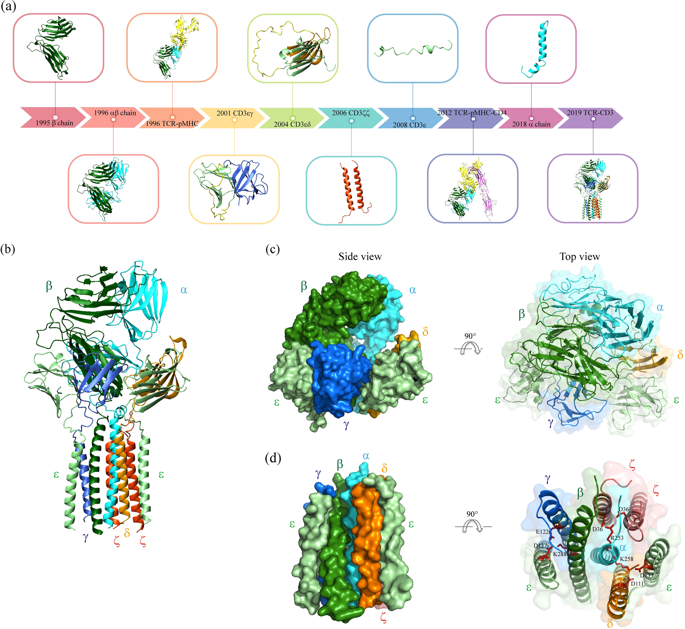
The T cell receptor (TCR) is one of the most complicated receptors in mammalian cells, and its triggering mechanism remains mysterious. As an octamer complex, TCR comprises an antigen-binding subunit (TCRαβ) and three CD3 signaling subunits (CD3ζζ, CD3δε, and CD3γε). Engagement of TCRαβ with an antigen peptide presented on the MHC leads to tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) in CD3 cytoplasmic domains (CDs), thus translating extracellular binding kinetics to intracellular signaling events. Whether conformational change plays an important role in the transmembrane signal transduction of TCR is under debate. Attracted by the complexity and functional importance of TCR, many groups have been studying TCR structure and triggering for decades using diverse biochemical and biophysical tools. Here, we synthesize these structural studies and discuss the relevance of the conformational change model in TCR triggering.
中文翻译:

T 细胞受体触发的结构理解。
T细胞受体(TCR)是哺乳动物细胞中最复杂的受体之一,其触发机制仍然是个谜。作为八聚体复合物,TCR 包含一个抗原结合亚基 (TCRαβ) 和三个 CD3 信号亚基(CD3δε、CD3δε 和 CD3γε)。 TCRαβ 与 MHC 上呈递的抗原肽结合,导致 CD3 胞质结构域 (CD) 中基于免疫受体酪氨酸的激活基序 (ITAM) 的酪氨酸磷酸化,从而将细胞外结合动力学转化为细胞内信号传导事件。构象变化是否在 TCR 跨膜信号转导中发挥重要作用仍存在争议。受 TCR 的复杂性和功能重要性的吸引,许多团队几十年来一直在使用不同的生化和生物物理工具研究 TCR 结构和触发。在这里,我们综合了这些结构研究,并讨论了构象变化模型在 TCR 触发中的相关性。
更新日期:2020-02-11
中文翻译:

T 细胞受体触发的结构理解。
T细胞受体(TCR)是哺乳动物细胞中最复杂的受体之一,其触发机制仍然是个谜。作为八聚体复合物,TCR 包含一个抗原结合亚基 (TCRαβ) 和三个 CD3 信号亚基(CD3δε、CD3δε 和 CD3γε)。 TCRαβ 与 MHC 上呈递的抗原肽结合,导致 CD3 胞质结构域 (CD) 中基于免疫受体酪氨酸的激活基序 (ITAM) 的酪氨酸磷酸化,从而将细胞外结合动力学转化为细胞内信号传导事件。构象变化是否在 TCR 跨膜信号转导中发挥重要作用仍存在争议。受 TCR 的复杂性和功能重要性的吸引,许多团队几十年来一直在使用不同的生化和生物物理工具研究 TCR 结构和触发。在这里,我们综合了这些结构研究,并讨论了构象变化模型在 TCR 触发中的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号