当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tariquidar-related triazoles as potent, selective and stable inhibitors of ABCG2 (BCRP).
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.ejmech.2020.112133 Frauke Antoni 1 , Manuel Bause 2 , Matthias Scholler 1 , Stefanie Bauer 1 , Simone A Stark 2 , Scott M Jackson 3 , Ioannis Manolaridis 3 , Kaspar P Locher 3 , Burkhard König 2 , Armin Buschauer 1 , Günther Bernhardt 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.ejmech.2020.112133 Frauke Antoni 1 , Manuel Bause 2 , Matthias Scholler 1 , Stefanie Bauer 1 , Simone A Stark 2 , Scott M Jackson 3 , Ioannis Manolaridis 3 , Kaspar P Locher 3 , Burkhard König 2 , Armin Buschauer 1 , Günther Bernhardt 1
Affiliation
|
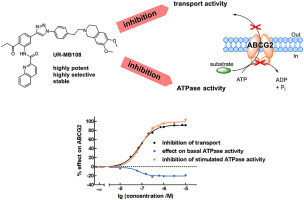
Tariquidar derivatives have been described as potent and selective ABCG2 inhibitors. However, their susceptibility to hydrolysis limits their applicability. The current study comprises the synthesis and characterization of novel tariquidar-related inhibitors, obtained by bioisosteric replacement of the labile moieties in our previous tariquidar analog UR-ME22-1 (9). CuAAC ("click" reaction) gave convenient access to a triazole core as a substitute for the labile amide group and the labile ester moiety was replaced by different acyl groups in a Sugasawa reaction. A stability assay proved the enhancement of the stability in blood plasma. Compounds UR-MB108 (57) and UR-MB136 (59) inhibited ABCG2 in a Hoechst 33342 transport assay with an IC50 value of about 80 nM and belong to the most potent ABCG2 inhibitors described so far. Compound 57 was highly selective, whereas its PEGylated analog 59 showed some potency at ABCB1. Both 57 and 59 produced an ABCG2 ATPase-depressing effect which is in agreement with our precedent cryo-EM study identifying 59 as an ATPase inhibitor that exerts its effect via locking the inward-facing conformation. Thermostabilization of ABCG2 by 57 and 59 can be taken as a hint to comparable binding to ABCG2. As reference substances, compounds 57 and 59 allow additional mechanistic studies on ABCG2 inhibition. Due to their stability in blood plasma, they are also applicable in vivo. The highly specific inhibitor 57 is suited for PET labeling, helping to further elucidate the (patho)physiological role of ABCG2, e.g. at the BBB.
中文翻译:

塔里基达(Tariquidar)相关的三唑是ABCG2(BCRP)的有效,选择性和稳定抑制剂。
塔里基达衍生物已被描述为有效的和选择性的ABCG2抑制剂。但是,它们对水解的敏感性限制了它们的适用性。目前的研究包括新型的与tariquidar相关的抑制剂的合成和表征,这些抑制剂是通过在我们先前的tariquidar类似物UR-ME22-1(9)中进行生物等位取代不稳定部分而获得的。CuAAC(“点击”反应)使三唑核方便地用作不稳定酰胺基的替代物,并且在Sugasawa反应中,不稳定酯部分被不同的酰基取代。稳定性测定证明了血浆中稳定性的增强。在Hoechst 33342转运分析中,化合物UR-MB108(57)和UR-MB136(59)抑制ABCG2,IC50值约为80 nM,属于迄今为止描述的最有效的ABCG2抑制剂。化合物57具有高选择性,而其PEG化的类似物59在ABCB1处显示出一定的效力。57和59均产生了降低ABCG2 ATPase的作用,这与我们先前的冷冻-EM研究一致,后者确定59是通过锁定向内构象发挥作用的ATPase抑制剂。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高度特异性的抑制剂57适用于PET标记,有助于进一步阐明ABCG2(例如在BBB上)的(病理)生理作用。57和59均产生了降低ABCG2 ATPase的作用,这与我们先前的冷冻EM研究一致,后者确定59是通过锁定向内构象发挥作用的ATPase抑制剂。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高度特异性的抑制剂57适用于PET标记,有助于进一步阐明ABCG2(例如在BBB上)的(病理)生理作用。57和59均产生了降低ABCG2 ATPase的作用,这与我们先前的冷冻EM研究一致,后者确定59是通过锁定向内构象发挥作用的ATPase抑制剂。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高特异性抑制剂57适用于PET标记,有助于进一步阐明ABCG2的(病理)生理作用,例如在BBB处。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高度特异性的抑制剂57适用于PET标记,有助于进一步阐明ABCG2(例如在BBB上)的(病理)生理作用。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高度特异性的抑制剂57适用于PET标记,有助于进一步阐明ABCG2(例如在BBB上)的(病理)生理作用。
更新日期:2020-02-10
中文翻译:

塔里基达(Tariquidar)相关的三唑是ABCG2(BCRP)的有效,选择性和稳定抑制剂。
塔里基达衍生物已被描述为有效的和选择性的ABCG2抑制剂。但是,它们对水解的敏感性限制了它们的适用性。目前的研究包括新型的与tariquidar相关的抑制剂的合成和表征,这些抑制剂是通过在我们先前的tariquidar类似物UR-ME22-1(9)中进行生物等位取代不稳定部分而获得的。CuAAC(“点击”反应)使三唑核方便地用作不稳定酰胺基的替代物,并且在Sugasawa反应中,不稳定酯部分被不同的酰基取代。稳定性测定证明了血浆中稳定性的增强。在Hoechst 33342转运分析中,化合物UR-MB108(57)和UR-MB136(59)抑制ABCG2,IC50值约为80 nM,属于迄今为止描述的最有效的ABCG2抑制剂。化合物57具有高选择性,而其PEG化的类似物59在ABCB1处显示出一定的效力。57和59均产生了降低ABCG2 ATPase的作用,这与我们先前的冷冻-EM研究一致,后者确定59是通过锁定向内构象发挥作用的ATPase抑制剂。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高度特异性的抑制剂57适用于PET标记,有助于进一步阐明ABCG2(例如在BBB上)的(病理)生理作用。57和59均产生了降低ABCG2 ATPase的作用,这与我们先前的冷冻EM研究一致,后者确定59是通过锁定向内构象发挥作用的ATPase抑制剂。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高度特异性的抑制剂57适用于PET标记,有助于进一步阐明ABCG2(例如在BBB上)的(病理)生理作用。57和59均产生了降低ABCG2 ATPase的作用,这与我们先前的冷冻EM研究一致,后者确定59是通过锁定向内构象发挥作用的ATPase抑制剂。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高特异性抑制剂57适用于PET标记,有助于进一步阐明ABCG2的(病理)生理作用,例如在BBB处。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高度特异性的抑制剂57适用于PET标记,有助于进一步阐明ABCG2(例如在BBB上)的(病理)生理作用。ABCG2通过57和59的热稳定作用可被认为是与ABCG2相当结合的暗示。作为参考物质,化合物57和59可以进行ABCG2抑制的其他机理研究。由于它们在血浆中的稳定性,它们也可以在体内使用。高度特异性的抑制剂57适用于PET标记,有助于进一步阐明ABCG2(例如在BBB上)的(病理)生理作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号