当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Negative allosteric modulators of the GluN2B NMDA receptor with phenylethylamine structure embedded in ring-expanded and ring-contracted scaffolds.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.ejmech.2020.112138 Louisa Temme 1 , Elena Bechthold 2 , Julian A Schreiber 3 , Sandeep Gawaskar 1 , Dirk Schepmann 2 , Dina Robaa 4 , Wolfgang Sippl 4 , Guiscard Seebohm 5 , Bernhard Wünsch 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.ejmech.2020.112138 Louisa Temme 1 , Elena Bechthold 2 , Julian A Schreiber 3 , Sandeep Gawaskar 1 , Dirk Schepmann 2 , Dina Robaa 4 , Wolfgang Sippl 4 , Guiscard Seebohm 5 , Bernhard Wünsch 1
Affiliation
|
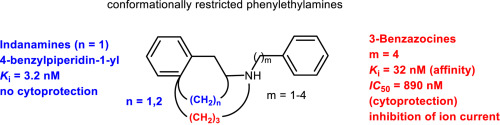
A set of GluN2B NMDA receptor antagonists with conformationally restricted phenylethylamine substructure was prepared and pharmacologically evaluated. The phenylethylamine substructure was embedded in ring expanded 3-benzazocines 4 as well as ring-contracted tetralinamines 6 and indanamines 7. The ligands 4, 6 and 7 were synthesized by reductive alkylation of secondary amine 11, reductive amination of ketones 12 and 16 and nucleophilic substitution of nosylates 14 and 17. The moderate GluN2B affinity of 3-benzazocine 4d (Ki = 32 nM) translated into moderate cytoprotective activity (IC50 = 890 nM) and moderate ion channel inhibition (60% at 10 μM) in two-electrode voltage clamp experiments with GluN1a/GluN2B expressing oocytes. Although some of the tetralinamines 6 and indanamines 7 showed very high GluN2B affinity (e.g. Ki (7f) = 3.2 nM), they could not inhibit glutamate/glycine inducted cytotoxicity. The low cytoprotective activity of 3-benzazocines 4, tetralinamines 6 and indanamines 7 was attributed to the missing OH moiety at the benzene ring and/or in benzylic position. Docking studies showed that the novel GluN2B ligands adopt similar binding poses as Ro 25-6981 with the central H-bond interaction between the protonated amino moiety of the ligands and the carbamoyl moiety of Gln110. However, due to the lack of a second H-bond forming group, the ligands can adopt two binding poses within the ifenprodil binding pocket.
中文翻译:

负的GluN2B NMDA受体的变构调节剂,其苯乙胺结构嵌入环扩展和环收缩的支架中。
制备了一组具有构象受限的苯乙胺亚结构的GluN2B NMDA受体拮抗剂,并进行了药理学评估。苯乙胺的亚结构包埋在扩环的3-苯甲唑啉4和缩合的四氮杂胺6和茚满胺7中。配体4、6和7是通过仲胺11的还原烷基化,酮12和16的还原胺化以及亲核性合成的Nosylates 14和17的取代。3-benzazocine 4d(Ki = 32 nM)的中等GluN2B亲和力在两电极电压下转化为中等的细胞保护活性(IC50 = 890 nM)和中等的离子通道抑制(10%时为60%)。表达GluN1a / GluN2B的卵母细胞的钳位实验。尽管某些四氢化萘胺6和茚满胺7具有很高的GluN2B亲和力(例如Ki(7f)= 3.2 nM),它们不能抑制谷氨酸/甘氨酸诱导的细胞毒性。3-苯甲唑啉4,四氢萘胺6和茚满胺7的低细胞保护活性归因于苯环和/或苄基位置上缺少的OH部分。对接研究表明,新型GluN2B配体采用与Ro 25-6981类似的结合姿势,且配体的质子化氨基部分与Gln110的氨基甲酰基部分之间具有中心H键相互作用。然而,由于缺乏第二个H键形成基团,配体可在艾芬地尔结合袋内采用两个结合姿势。对接研究表明,新型GluN2B配体采用与Ro 25-6981类似的结合姿势,且配体的质子化氨基部分与Gln110的氨基甲酰基部分之间具有中心H键相互作用。然而,由于缺乏第二个H键形成基团,配体可在艾芬地尔结合袋内采用两个结合姿势。对接研究表明,新型GluN2B配体采用与Ro 25-6981类似的结合姿势,且配体的质子化氨基部分与Gln110的氨基甲酰基部分之间具有中心H键相互作用。然而,由于缺乏第二个H键形成基团,配体可在艾芬地尔结合袋内采用两个结合姿势。
更新日期:2020-02-10
中文翻译:

负的GluN2B NMDA受体的变构调节剂,其苯乙胺结构嵌入环扩展和环收缩的支架中。
制备了一组具有构象受限的苯乙胺亚结构的GluN2B NMDA受体拮抗剂,并进行了药理学评估。苯乙胺的亚结构包埋在扩环的3-苯甲唑啉4和缩合的四氮杂胺6和茚满胺7中。配体4、6和7是通过仲胺11的还原烷基化,酮12和16的还原胺化以及亲核性合成的Nosylates 14和17的取代。3-benzazocine 4d(Ki = 32 nM)的中等GluN2B亲和力在两电极电压下转化为中等的细胞保护活性(IC50 = 890 nM)和中等的离子通道抑制(10%时为60%)。表达GluN1a / GluN2B的卵母细胞的钳位实验。尽管某些四氢化萘胺6和茚满胺7具有很高的GluN2B亲和力(例如Ki(7f)= 3.2 nM),它们不能抑制谷氨酸/甘氨酸诱导的细胞毒性。3-苯甲唑啉4,四氢萘胺6和茚满胺7的低细胞保护活性归因于苯环和/或苄基位置上缺少的OH部分。对接研究表明,新型GluN2B配体采用与Ro 25-6981类似的结合姿势,且配体的质子化氨基部分与Gln110的氨基甲酰基部分之间具有中心H键相互作用。然而,由于缺乏第二个H键形成基团,配体可在艾芬地尔结合袋内采用两个结合姿势。对接研究表明,新型GluN2B配体采用与Ro 25-6981类似的结合姿势,且配体的质子化氨基部分与Gln110的氨基甲酰基部分之间具有中心H键相互作用。然而,由于缺乏第二个H键形成基团,配体可在艾芬地尔结合袋内采用两个结合姿势。对接研究表明,新型GluN2B配体采用与Ro 25-6981类似的结合姿势,且配体的质子化氨基部分与Gln110的氨基甲酰基部分之间具有中心H键相互作用。然而,由于缺乏第二个H键形成基团,配体可在艾芬地尔结合袋内采用两个结合姿势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号