当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 4-(2-fluorophenyl)-7-methoxycoumarin: experimental and computational evidence for intramolecular and intermolecular C-F···H-C bonds.
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-02-10 , DOI: 10.3762/bjoc.16.22 Vuyisa Mzozoyana 1 , Fanie R van Heerden 1 , Craig Grimmer 1
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-02-10 , DOI: 10.3762/bjoc.16.22 Vuyisa Mzozoyana 1 , Fanie R van Heerden 1 , Craig Grimmer 1
Affiliation
|
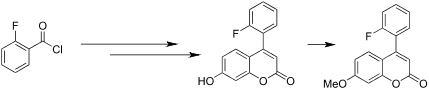
4-(2-Fluorophenyl)-7-methoxycoumarin (6) was synthesized by Pechmann reaction under mild conditions via a three-step reaction. The solution-state 1H NMR spectra of 6 showed a strong intramolecular interaction between F and H5 (J FH = 2.6 Hz) and 13C NMR suggested that this C-F···H-C coupling is a through-space interaction. The 2D 19F-{1H} HOESY and 1H-{19F} 1D experiments were done to confirm this F···H interaction. The single crystal X-ray structure and the DFT-optimized structure showed that the fluorinated phenyl ring favors the orientation with the fluorine atom closer to H5 than H3. The X-ray structure also showed the existence of the intermolecular C-F···H-C interaction.
中文翻译:

4-(2-氟苯基)-7-甲氧基香豆素的合成:分子内和分子间CF···HC键的实验和计算证据。
通过Pechmann反应在温和条件下通过三步反应合成4-(2-氟苯基)-7-甲氧基香豆素(6)。6的溶液态1H NMR光谱显示F和H5之间存在强分子内相互作用(J FH = 2.6 Hz),13 C NMR表明这种CF··HC偶联是一种空间相互作用。进行了2D 19F- {1H} HOESY和1H- {19F} 1D实验以确认这种F··H相互作用。单晶X射线结构和DFT优化结构表明,氟化苯环有利于氟原子比H3更靠近H5的取向。X射线结构也表明存在分子间CF···HC相互作用。
更新日期:2020-02-10
中文翻译:

4-(2-氟苯基)-7-甲氧基香豆素的合成:分子内和分子间CF···HC键的实验和计算证据。
通过Pechmann反应在温和条件下通过三步反应合成4-(2-氟苯基)-7-甲氧基香豆素(6)。6的溶液态1H NMR光谱显示F和H5之间存在强分子内相互作用(J FH = 2.6 Hz),13 C NMR表明这种CF··HC偶联是一种空间相互作用。进行了2D 19F- {1H} HOESY和1H- {19F} 1D实验以确认这种F··H相互作用。单晶X射线结构和DFT优化结构表明,氟化苯环有利于氟原子比H3更靠近H5的取向。X射线结构也表明存在分子间CF···HC相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号