当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The engineering of stilbazolium/iodocuprate hybrids with optical/electrical performances by modulating inter-molecular charge transfer among H-aggregated chromophores
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020/02/10 , DOI: 10.1039/c9qi01672d Wei Wu 1, 2, 3, 4 , Xiang-Ling Lin 1, 2, 3, 4 , Qian Liu 1, 2, 3, 4 , Yan He 1, 2, 3, 4 , You-Ren Huang 1, 2, 3, 4 , Bin Chen 1, 2, 3, 4 , Hao-Hong Li 1, 2, 3, 4 , Zhi-Rong Chen 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020/02/10 , DOI: 10.1039/c9qi01672d Wei Wu 1, 2, 3, 4 , Xiang-Ling Lin 1, 2, 3, 4 , Qian Liu 1, 2, 3, 4 , Yan He 1, 2, 3, 4 , You-Ren Huang 1, 2, 3, 4 , Bin Chen 1, 2, 3, 4 , Hao-Hong Li 1, 2, 3, 4 , Zhi-Rong Chen 1, 2, 3, 4
Affiliation
|
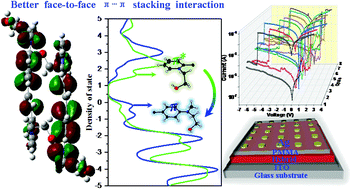
The combination of D–π-A stilbazolium-type chromophores bearing ortho-N-alkyl substituents with iodocuprate resulted in three novel hybrids, these were, [(CMAMP)2(CuI3)(acetone)0.5]n (1), [(HMAMP)(Cu3I4)]n (2), and [(HMAHP)2(Cu5I7)]n (3), which present natural quantum-well architectures with void spaces of quasi-2-D organic layers occupied by iodocuprate anions via C–H⋯I hydrogen bonds. The D–π-A stilbazolium dyes exhibit head-to-tail arranged H-aggregations with face-to-face π⋯π stacking interactions. Theoretical calculations suggest that the charge densities on the benzenes can be strengthened from the electron-withdrawing group (–CN) to the electron-donating group (–OH), and the –C2H5OH substituents on the ortho-N of the pyridines not only help to open up the band gaps, but also provide a steric effect for better face-to-face π⋯π stacking interactions. Therefore, HMAHP+ in 3 possesses the strongest face-to-face π⋯π stacking interactions owing to the presence of a longer –C2H5OH group in the ortho-N position, and consequently, blue-shifted photoluminescence, a stronger photocurrent (0.28 mA), and a higher ON/OFF ratio (1.6 × 104) can be observed in 3. In particular, electrical bistability performances can be observed in ITO/hybrids/poly (methyl methacrylate) (PMMA)/Ag devices, which are explained using Schottky emission, space-charge-limited current effect (SCLC) and Ohmic mechanisms. According to theoretical calculations, the band gap switching from the semi-conductor to the conductor (high resistance state (HRS) to low resistance state (LRS)) after trapping electrons is exclusively dominated by the π bonding and anti-bonding orbitals of the stilbazolium dyes, which indicates a future direction for the design of stilbazolium-containing memory devices.
中文翻译:

通过调节H聚集的生色团之间的分子间电荷转移来设计具有光/电性能的stilbazolium / iodocuprate杂化体
带有邻N-烷基取代基的D–π-A噻唑类发色团与碘多普酸盐的结合产生了三种新型杂种,它们是[(CMAMP)2(CuI 3)(丙酮)0.5 ] n(1),[ (HMAMP)(Cu 3 I 4)] n(2)和[(HMAHP)2(Cu 5 I 7)] n(3),它们呈现出具有准2- D有机空隙的天然量子阱结构通过iodocuprate阴离子占据的层通过C–H⋯I氢键。D-π-Astilbazolium染料具有从头到尾排列的H聚集体,且具有面对面的π⋯π堆积相互作用。理论计算表明,从吸电子基团(-CN)到给电子基团(-OH)可以增强苯上的电荷密度,并且邻位-N上的-C 2 H 5 OH取代基可以增强。吡啶不仅有助于打开带隙,而且还提供空间位阻效应,以实现更好的面对面π⋯π堆叠相互作用。因此,HMAHP +在3具有最强的面到面π⋯π堆叠由于较长-C存在相互作用2 ħ 5 OH基团在邻位-N位置,因此,在3中可以观察到蓝移的光致发光,更强的光电流(0.28 mA)和更高的开/关比(1.6×10 4)。尤其是,可以在ITO /混合材料/聚(甲基丙烯酸甲酯)(PMMA)/ Ag器件中观察到电双稳态性能,这可以使用肖特基发射,空间电荷限制电流效应(SCLC)和欧姆机制进行解释。根据理论计算,俘获电子后从半导体到导体的带隙切换(高阻态(HRS)到低阻态(LRS))仅由the的π键和反键轨道控制染料,这指示了含有stilbazolium的存储设备设计的未来方向。
更新日期:2020-03-19
中文翻译:

通过调节H聚集的生色团之间的分子间电荷转移来设计具有光/电性能的stilbazolium / iodocuprate杂化体
带有邻N-烷基取代基的D–π-A噻唑类发色团与碘多普酸盐的结合产生了三种新型杂种,它们是[(CMAMP)2(CuI 3)(丙酮)0.5 ] n(1),[ (HMAMP)(Cu 3 I 4)] n(2)和[(HMAHP)2(Cu 5 I 7)] n(3),它们呈现出具有准2- D有机空隙的天然量子阱结构通过iodocuprate阴离子占据的层通过C–H⋯I氢键。D-π-Astilbazolium染料具有从头到尾排列的H聚集体,且具有面对面的π⋯π堆积相互作用。理论计算表明,从吸电子基团(-CN)到给电子基团(-OH)可以增强苯上的电荷密度,并且邻位-N上的-C 2 H 5 OH取代基可以增强。吡啶不仅有助于打开带隙,而且还提供空间位阻效应,以实现更好的面对面π⋯π堆叠相互作用。因此,HMAHP +在3具有最强的面到面π⋯π堆叠由于较长-C存在相互作用2 ħ 5 OH基团在邻位-N位置,因此,在3中可以观察到蓝移的光致发光,更强的光电流(0.28 mA)和更高的开/关比(1.6×10 4)。尤其是,可以在ITO /混合材料/聚(甲基丙烯酸甲酯)(PMMA)/ Ag器件中观察到电双稳态性能,这可以使用肖特基发射,空间电荷限制电流效应(SCLC)和欧姆机制进行解释。根据理论计算,俘获电子后从半导体到导体的带隙切换(高阻态(HRS)到低阻态(LRS))仅由the的π键和反键轨道控制染料,这指示了含有stilbazolium的存储设备设计的未来方向。









































 京公网安备 11010802027423号
京公网安备 11010802027423号