当前位置:
X-MOL 学术
›
JAMA Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of Integrated Outpatient Palliative Care With Standard Care in Patients With Parkinson Disease and Related Disorders: A Randomized Clinical Trial.
JAMA Neurology ( IF 20.4 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamaneurol.2019.4992 Benzi M Kluger 1, 2 , Janis Miyasaki 3 , Maya Katz 4 , Nicholas Galifianakis 4 , Kirk Hall 1 , Steven Pantilat 5 , Ryan Khan 1 , Cari Friedman 1 , Wendy Cernik 1 , Yuika Goto 5 , Judith Long 4 , Diane Fairclough 6 , Stefan Sillau 1 , Jean S Kutner 7
JAMA Neurology ( IF 20.4 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamaneurol.2019.4992 Benzi M Kluger 1, 2 , Janis Miyasaki 3 , Maya Katz 4 , Nicholas Galifianakis 4 , Kirk Hall 1 , Steven Pantilat 5 , Ryan Khan 1 , Cari Friedman 1 , Wendy Cernik 1 , Yuika Goto 5 , Judith Long 4 , Diane Fairclough 6 , Stefan Sillau 1 , Jean S Kutner 7
Affiliation
|
Importance
Parkinson disease and related disorders (PDRD) have consequences for quality of life (QoL) and are the 14th leading cause of death in the United States. Despite growing interest in palliative care (PC) for persons with PDRD, few studies are available supporting its effectiveness.
Objective
To determine if outpatient PC is associated with improvements in patient-centered outcomes compared with standard care among patients with PDRD and their caregivers.
Design, Setting, and Participants
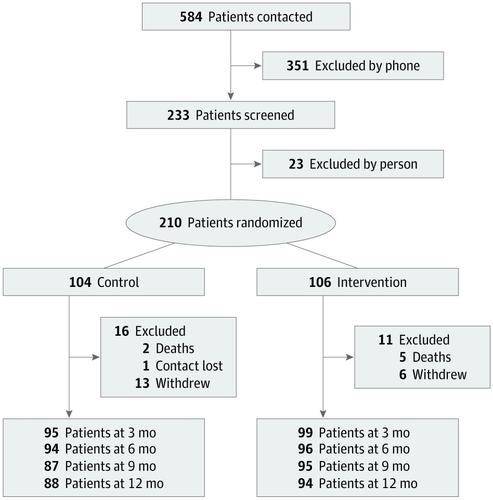
This randomized clinical trial enrolled participants at 3 academic tertiary care centers between November 1, 2015, and September 30, 2017, and followed them up for 1 year. A total of 584 persons with PDRD were referred to the study. Of those, 351 persons were excluded by phone and 23 were excluded during in-person screenings. Patients were eligible to participate if they had PDRD and moderate to high PC needs. Patients were excluded if they had urgent PC needs, another diagnosis meriting PC, were already receiving PC, or were unable or unwilling to follow the study protocol. Enrolled participants were assigned to receive standard care plus outpatient integrated PC or standard care alone. Data were analyzed between November 1, 2018, and December 9, 2019.
Interventions
Outpatient integrated PC administered by a neurologist, social worker, chaplain, and nurse using PC checklists, with guidance and selective involvement from a palliative medicine specialist. Standard care was provided by a neurologist and a primary care practitioner.
Main Outcomes and Measures
The primary outcomes were the differences in patient quality of life (QoL; measured by the Quality of Life in Alzheimer Disease scale) and caregiver burden (measured by the Zarit Burden Interview) between the PC intervention and standard care groups at 6 months.
Results
A total of 210 patients with PDRD (135 men [64.3%]; mean [SD] age, 70.1 [8.2] years) and 175 caregivers (128 women [73.1%]; mean [SD] age, 66.1 [11.1] years) were enrolled in the study; 193 participants (91.9%) were white and non-Hispanic. Compared with participants receiving standard care alone at 6 months, participants receiving the PC intervention had better QoL (mean [SD], 0.66 [5.5] improvement vs 0.84 [4.2] worsening; treatment effect estimate, 1.87; 95% CI, 0.47-3.27; P = .009). No significant difference was observed in caregiver burden (mean [SD], 2.3 [5.0] improvement vs 1.2 [5.6] improvement in the standard care group; treatment effect estimate, -1.62; 95% CI, -3.32 to 0.09; P = .06). Other significant differences favoring the PC intervention included nonmotor symptom burden, motor symptom severity, completion of advance directives, caregiver anxiety, and caregiver burden at 12 months. No outcomes favored standard care alone. Secondary analyses suggested that benefits were greater for persons with higher PC needs.
Conclusions and Relevance
Outpatient PC is associated with benefits among patients with PDRD compared with standard care alone. This study supports efforts to integrate PC into PDRD care. The lack of diversity and implementation of PC at experienced centers suggests a need for implementation research in other populations and care settings.
Trial Registration
ClinicalTrials.gov Identifier: NCT02533921.
中文翻译:

帕金森病和相关疾病患者的综合门诊姑息治疗与标准治疗的比较:一项随机临床试验。
重要的帕金森氏病和相关疾病(PDRD)对生活质量(QoL)有影响,并且是美国第14大死亡原因。尽管人们对PDRD患者的姑息治疗(PC)越来越感兴趣,但很少有研究支持其有效性。目的确定与PDRD及其护理人员的标准护理相比,门诊PC是否与以患者为中心的结果改善相关。设计,设置和参与者这项随机临床试验在2015年11月1日至2017年9月30日期间在3个学术三级护理中心招募了参与者,并对他们进行了为期一年的随访。共有584名PDRD患者转入了研究。其中,有351人被电话排除在外,在进行现场筛查时有23人被排除在外。如果患者患有PDRD且中度至高PC需求,则有资格参加。如果患者有紧急的PC需求,另一个需要诊断的PC,已经接受PC或不能或不愿意遵循研究方案的患者,则被排除在外。入选的参与者被分配接受标准护理加门诊集成式PC或仅接受标准护理。在2018年11月1日至2019年12月9日之间对数据进行了分析。干预措施由神经科医生,社会工作者,牧师和护士使用PC核对表管理的门诊综合PC,并由姑息医学专家指导和选择性参与。神经科医生和初级保健医生提供了标准护理。主要结果和指标主要结果是患者生活质量的差异(QoL;PC干预组和标准护理组在6个月时的生活质量(按阿尔茨海默氏病患者的生活质量量表)和照料者负担(按Zarit Burden访谈测量)。结果共有210名PDRD患者(135名男性[64.3%];平均[SD]年龄,70.1 [8.2]岁)和175名护理人员(128名女性[73.1%];平均[SD]年龄,66.1 [11.1]岁) )被纳入研究;193名参与者(91.9%)是白人和非西班牙裔。与仅接受6个月标准护理的参与者相比,接受PC干预的参与者的QoL更好(平均[SD]改善为0.66 [5.5],恶化为0.84 [4.2];治疗效果估计为1.87; 95%CI为0.47-3.27) ; P = .009)。照料者负担没有观察到显着差异(平均[SD]改善2.3 [5.0],而标准护理组改善1.2 [5.6];治疗效果估计值为-1.62;95%CI,-3.32至0.09;P = .06)。其他有利于PC干预的显着差异包括非运动症状负担,运动症状严重程度,完成预先指示,护理者焦虑和12个月护理者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论和相关性与仅标准护理相比,PDRD患者的门诊PC与获益有关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。其他有利于PC干预的显着差异包括非运动症状负担,运动症状严重程度,完成预先指示,护理者焦虑和12个月护理者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论和相关性与仅标准护理相比,PDRD患者的门诊PC与获益有关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。其他有利于PC干预的显着差异包括非运动症状负担,运动症状严重程度,完成预先指示,护理者焦虑和12个月护理者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论与相关性与仅标准治疗相比,PDRD患者的门诊PC与收益相关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。在12个月内完成预先指示,照顾者焦虑和照顾者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论和相关性与仅标准护理相比,PDRD患者的门诊PC与获益有关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。在12个月时完成预先指示,照顾者焦虑和照顾者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论与相关性与仅标准治疗相比,PDRD患者的门诊PC与收益相关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。结论与相关性与仅标准治疗相比,PDRD患者的门诊PC与收益相关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。结论与相关性与单独标准治疗相比,PDRD患者的门诊PC与收益相关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。
更新日期:2020-05-01
中文翻译:

帕金森病和相关疾病患者的综合门诊姑息治疗与标准治疗的比较:一项随机临床试验。
重要的帕金森氏病和相关疾病(PDRD)对生活质量(QoL)有影响,并且是美国第14大死亡原因。尽管人们对PDRD患者的姑息治疗(PC)越来越感兴趣,但很少有研究支持其有效性。目的确定与PDRD及其护理人员的标准护理相比,门诊PC是否与以患者为中心的结果改善相关。设计,设置和参与者这项随机临床试验在2015年11月1日至2017年9月30日期间在3个学术三级护理中心招募了参与者,并对他们进行了为期一年的随访。共有584名PDRD患者转入了研究。其中,有351人被电话排除在外,在进行现场筛查时有23人被排除在外。如果患者患有PDRD且中度至高PC需求,则有资格参加。如果患者有紧急的PC需求,另一个需要诊断的PC,已经接受PC或不能或不愿意遵循研究方案的患者,则被排除在外。入选的参与者被分配接受标准护理加门诊集成式PC或仅接受标准护理。在2018年11月1日至2019年12月9日之间对数据进行了分析。干预措施由神经科医生,社会工作者,牧师和护士使用PC核对表管理的门诊综合PC,并由姑息医学专家指导和选择性参与。神经科医生和初级保健医生提供了标准护理。主要结果和指标主要结果是患者生活质量的差异(QoL;PC干预组和标准护理组在6个月时的生活质量(按阿尔茨海默氏病患者的生活质量量表)和照料者负担(按Zarit Burden访谈测量)。结果共有210名PDRD患者(135名男性[64.3%];平均[SD]年龄,70.1 [8.2]岁)和175名护理人员(128名女性[73.1%];平均[SD]年龄,66.1 [11.1]岁) )被纳入研究;193名参与者(91.9%)是白人和非西班牙裔。与仅接受6个月标准护理的参与者相比,接受PC干预的参与者的QoL更好(平均[SD]改善为0.66 [5.5],恶化为0.84 [4.2];治疗效果估计为1.87; 95%CI为0.47-3.27) ; P = .009)。照料者负担没有观察到显着差异(平均[SD]改善2.3 [5.0],而标准护理组改善1.2 [5.6];治疗效果估计值为-1.62;95%CI,-3.32至0.09;P = .06)。其他有利于PC干预的显着差异包括非运动症状负担,运动症状严重程度,完成预先指示,护理者焦虑和12个月护理者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论和相关性与仅标准护理相比,PDRD患者的门诊PC与获益有关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。其他有利于PC干预的显着差异包括非运动症状负担,运动症状严重程度,完成预先指示,护理者焦虑和12个月护理者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论和相关性与仅标准护理相比,PDRD患者的门诊PC与获益有关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。其他有利于PC干预的显着差异包括非运动症状负担,运动症状严重程度,完成预先指示,护理者焦虑和12个月护理者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论与相关性与仅标准治疗相比,PDRD患者的门诊PC与收益相关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。在12个月内完成预先指示,照顾者焦虑和照顾者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论和相关性与仅标准护理相比,PDRD患者的门诊PC与获益有关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。在12个月时完成预先指示,照顾者焦虑和照顾者负担。没有结果支持单独的标准护理。二级分析表明,对具有较高PC需求的人的好处更大。结论与相关性与仅标准治疗相比,PDRD患者的门诊PC与收益相关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。结论与相关性与仅标准治疗相比,PDRD患者的门诊PC与收益相关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。结论与相关性与单独标准治疗相比,PDRD患者的门诊PC与收益相关。这项研究支持将PC纳入PDRD护理的努力。经验丰富的中心缺乏PC的多样性和实施,这表明需要在其他人群和医疗机构中进行实施研究。试验注册ClinicalTrials.gov标识符:NCT02533921。










































 京公网安备 11010802027423号
京公网安备 11010802027423号