Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Collagen-Immobilized Extracellular FRET Reporter for Visualizing Protease Activity Secreted by Living Cells.
ACS Sensors ( IF 8.2 ) Pub Date : 2020-02-21 , DOI: 10.1021/acssensors.9b01456 Hawon Lee 1 , Se-Jeong Kim 2 , Heungsoo Shin 2, 3 , Young-Pil Kim 1, 3, 4
ACS Sensors ( IF 8.2 ) Pub Date : 2020-02-21 , DOI: 10.1021/acssensors.9b01456 Hawon Lee 1 , Se-Jeong Kim 2 , Heungsoo Shin 2, 3 , Young-Pil Kim 1, 3, 4
Affiliation
|
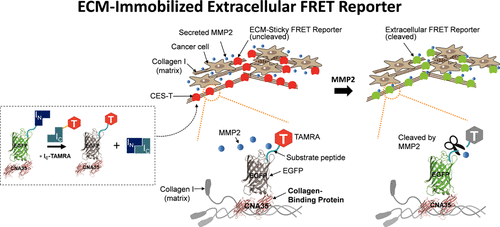
Despite the diverse roles of cell-secreted proteases in the extracellular matrix (ECM), classical methods to analyze protease activity have not been explored at the cell culture site. Here, we report a stable, matrix-sticky, and protease-sensitive extracellular reporter that comprises a collagen-binding protein and a Förster resonance energy transfer (FRET) coupler of an enhanced green fluorescent protein and a small dye molecule. The extracellular FRET reporter via split intein-mediated protein trans-splicing is able to adhere to collagen matrices, leading to fluorescence changes by matrix metalloproteinase-2 (MMP2) activity during living cell culture without impeding cell viability. When a proMMP2 mutant (Y581A) with altered protease secretion and activity was transfected into cancer cells, the reporter revealed a dramatic reduction in MMP2 activity in both two- and three-dimensional culture systems, compared with cells transfected with wild-type proMMP2. Our reporter is immediately amenable to monitor protease activity in diverse ECM-resident cells as well as to study protease-related extracellular signaling and tissue remodeling.
中文翻译:

胶原蛋白固定的细胞外FRET报告基因,用于观察活细胞分泌的蛋白酶活性。
尽管细胞分泌的蛋白酶在细胞外基质(ECM)中具有多种作用,但尚未在细胞培养位点探索分析蛋白酶活性的经典方法。在这里,我们报告了一个稳定的,对基质敏感的,对蛋白酶敏感的细胞外报道分子,该报道分子包括胶原蛋白结合蛋白和增强的绿色荧光蛋白和小的染料分子的Förster共振能量转移(FRET)偶联剂。通过分裂内含蛋白介导的蛋白质反式剪接,细胞外FRET报告基因能够粘附于胶原蛋白基质,在活细胞培养过程中通过基质金属蛋白酶2(MMP2)活性导致荧光变化,而不会阻碍细胞活力。当蛋白酶分泌和活性改变的proMMP2突变体(Y581A)转染到癌细胞中时,记者发现与转染野生型proMMP2的细胞相比,在二维和三维培养系统中MMP2的活性均大大降低。我们的记者立即可以监测多种ECM驻留细胞中的蛋白酶活性,以及研究蛋白酶相关的细胞外信号传导和组织重塑。
更新日期:2020-02-10
中文翻译:

胶原蛋白固定的细胞外FRET报告基因,用于观察活细胞分泌的蛋白酶活性。
尽管细胞分泌的蛋白酶在细胞外基质(ECM)中具有多种作用,但尚未在细胞培养位点探索分析蛋白酶活性的经典方法。在这里,我们报告了一个稳定的,对基质敏感的,对蛋白酶敏感的细胞外报道分子,该报道分子包括胶原蛋白结合蛋白和增强的绿色荧光蛋白和小的染料分子的Förster共振能量转移(FRET)偶联剂。通过分裂内含蛋白介导的蛋白质反式剪接,细胞外FRET报告基因能够粘附于胶原蛋白基质,在活细胞培养过程中通过基质金属蛋白酶2(MMP2)活性导致荧光变化,而不会阻碍细胞活力。当蛋白酶分泌和活性改变的proMMP2突变体(Y581A)转染到癌细胞中时,记者发现与转染野生型proMMP2的细胞相比,在二维和三维培养系统中MMP2的活性均大大降低。我们的记者立即可以监测多种ECM驻留细胞中的蛋白酶活性,以及研究蛋白酶相关的细胞外信号传导和组织重塑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号