当前位置:
X-MOL 学术
›
Adv. Sust. Syst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile Self‐Forming Superionic Conductors Based on Complex Borohydride Surface Oxidation
Advanced Sustainable Systems ( IF 6.5 ) Pub Date : 2020-02-09 , DOI: 10.1002/adsu.201900113 Xiaoxuan Luo 1 , Aditya Rawal 2 , Claudio Cazorla 3 , Kondo‐Francois Aguey‐Zinsou 1
Advanced Sustainable Systems ( IF 6.5 ) Pub Date : 2020-02-09 , DOI: 10.1002/adsu.201900113 Xiaoxuan Luo 1 , Aditya Rawal 2 , Claudio Cazorla 3 , Kondo‐Francois Aguey‐Zinsou 1
Affiliation
|
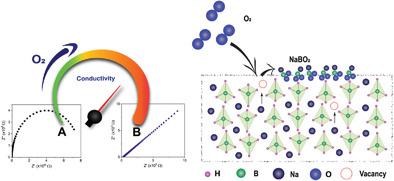
Complex hydrides have attracted significant attention as better inorganic solid‐state electrolytes owing to their lightweight and good compatibility with metal anodes (Li, Na, and/or Mg) for all solid‐state batteries. However, high ionic conductivity is usually observed at high temperatures upon the stabilization of adequate crystalline phases enabling fast ionic mobility. Here, an extremely simple strategy to significantly increase the ionic conductivity of complex borohydrides is reported. By exposing complex borohydrides to oxygen, the rearrangement of surface atoms upon the oxidation of borohydride particles and the resulting defects lead to extremely high ionic conductivity. NaBH4 and LiBH4 exposed to 5% O2 show an ionic conductivity of ≈10−3 S cm−1 at 35 °C. Similarly, oxidized Mg(BH4)2 displays a conductivity of ≈10−6 S cm−1 at 25 °C instead of 9.63 × 10−13 S cm−1. To the best of the authors' knowledge, this is to date, the simplest approach to tune the properties of borohydrides toward high ionic conductivity at room temperature as it does not rely on the difficult synthesis of large cage boron based anions to substitute BH4− and allow better ionic conduction paths. Owing its simplicity, the finding has the potential to enable new avenues toward the realization of viable complex borohydride based solid‐state electrolytes.
中文翻译:

基于复杂硼氢化物表面氧化的简便自形成超离子导体
复合氢化物由于重量轻且与所有固态电池的金属阳极(Li,Na和/或Mg)具有良好的相容性,因此作为更好的无机固态电解质已引起广泛关注。然而,通常在高温下观察到高的离子电导率,这是由于稳定了足够的结晶相而实现了快速的离子迁移率。在此,报道了一种极其简单的策略,可以显着提高复合硼氢化物的离子电导率。通过将复杂的硼氢化物暴露于氧气,硼氢化物颗粒氧化时表面原子的重排和所产生的缺陷导致极高的离子电导率。暴露于5%O 2的NaBH 4和LiBH 4的离子电导率约为10在35°C下为-3 S cm -1。类似地,氧化镁(BH 4)2所显示的≈10电导率-6小号厘米-1,在25℃,而不是9.63×10 -13小号厘米-1。为了最好的作者的知识,这是迄今为止,调整最简单的方法向高离子传导性硼氢化物在室温下,因为它不依赖于大笼子硼基阴离子难以合成替代BH性质4 -并允许更好的离子传导路径。由于其简单性,这一发现有可能为实现可行的基于硼氢化物的固态电解质提供新的途径。
更新日期:2020-03-05
中文翻译:

基于复杂硼氢化物表面氧化的简便自形成超离子导体
复合氢化物由于重量轻且与所有固态电池的金属阳极(Li,Na和/或Mg)具有良好的相容性,因此作为更好的无机固态电解质已引起广泛关注。然而,通常在高温下观察到高的离子电导率,这是由于稳定了足够的结晶相而实现了快速的离子迁移率。在此,报道了一种极其简单的策略,可以显着提高复合硼氢化物的离子电导率。通过将复杂的硼氢化物暴露于氧气,硼氢化物颗粒氧化时表面原子的重排和所产生的缺陷导致极高的离子电导率。暴露于5%O 2的NaBH 4和LiBH 4的离子电导率约为10在35°C下为-3 S cm -1。类似地,氧化镁(BH 4)2所显示的≈10电导率-6小号厘米-1,在25℃,而不是9.63×10 -13小号厘米-1。为了最好的作者的知识,这是迄今为止,调整最简单的方法向高离子传导性硼氢化物在室温下,因为它不依赖于大笼子硼基阴离子难以合成替代BH性质4 -并允许更好的离子传导路径。由于其简单性,这一发现有可能为实现可行的基于硼氢化物的固态电解质提供新的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号