当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organo-catalyzed asymmetric cascade annulation reaction for the construction of bi-spirocyclic pyrazolone and oxindole derivatives
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/07 , DOI: 10.1039/d0qo00001a Bing-Bing Sun 1, 2, 3, 4, 5 , Jun-Bo Chen 1, 2, 3, 4, 5 , Jun-Qi Zhang 1, 2, 3, 4, 5 , Xiao-Peng Yang 1, 2, 3, 4, 5 , Hao-Peng Lv 1, 2, 3, 4, 5 , Zheng Wang 6, 7, 8, 9, 10 , Xing-Wang Wang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/07 , DOI: 10.1039/d0qo00001a Bing-Bing Sun 1, 2, 3, 4, 5 , Jun-Bo Chen 1, 2, 3, 4, 5 , Jun-Qi Zhang 1, 2, 3, 4, 5 , Xiao-Peng Yang 1, 2, 3, 4, 5 , Hao-Peng Lv 1, 2, 3, 4, 5 , Zheng Wang 6, 7, 8, 9, 10 , Xing-Wang Wang 1, 2, 3, 4, 5
Affiliation
|
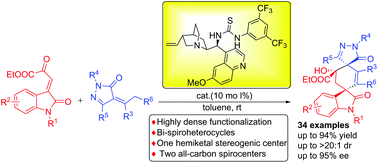
A bifunctional organocatalyst catalyzed tandem annulation reaction between β,γ-unsaturated α-ketoesters with α-arylidene pyrazolinones was reported. The tandem Michael–aldol process allows access to optically active bi-spirocyclic pyrazolone and oxindole compounds in high yields with good to excellent enantioselectivity and diastereoselectivity. The resulting bi-spirocyclic compounds possess highly dense functionalized substituents, two all-carbon spiro quaternary stereocenters and one hemiketal stereogenic centers.
中文翻译:

有机催化不对称级联环化反应用于双螺环吡唑啉酮和羟吲哚衍生物的构建
报道了在β,γ-不饱和α-酮酸酯与α-亚芳基吡唑啉酮之间的双官能有机催化剂催化的串联环化反应。串联的Michael–aldol工艺可以高收率获得旋光性双螺环吡唑啉酮和羟吲哚化合物,对映选择性和非对映选择性都非常好。所得的双螺环化合物具有高密度的官能化取代基,两个全碳螺环四元立体中心和一个半立体立体中心。
更新日期:2020-03-03
中文翻译:

有机催化不对称级联环化反应用于双螺环吡唑啉酮和羟吲哚衍生物的构建
报道了在β,γ-不饱和α-酮酸酯与α-亚芳基吡唑啉酮之间的双官能有机催化剂催化的串联环化反应。串联的Michael–aldol工艺可以高收率获得旋光性双螺环吡唑啉酮和羟吲哚化合物,对映选择性和非对映选择性都非常好。所得的双螺环化合物具有高密度的官能化取代基,两个全碳螺环四元立体中心和一个半立体立体中心。









































 京公网安备 11010802027423号
京公网安备 11010802027423号