当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Symmetrical and unsymmetrical fluorine-rich ullazines via controlled cycloaromatizations
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/05 , DOI: 10.1039/d0qo00033g Guoxian Zhang 1, 2, 3, 4 , Prabhat Gautam 1, 2, 3, 4 , Julian M. W. Chan 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/05 , DOI: 10.1039/d0qo00033g Guoxian Zhang 1, 2, 3, 4 , Prabhat Gautam 1, 2, 3, 4 , Julian M. W. Chan 1, 2, 3, 4
Affiliation
|
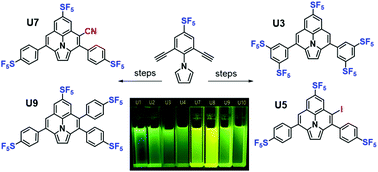
We report the synthesis of a series of fluorine-rich ullazines via well-controlled cycloaromatizations of highly electron-deficient alkynes. Specifically, three symmetrically-functionalized and three unsymmetrically-functionalized ullazine cores were constructed from 1-(2,6-dialkynylphenyl)-1H-pyrroles via controlled electrophilic cyclizations of highly electron-poor pentafluorosulfanylated (i.e., SF5-containing) phenylethynyl moieties onto the pyrrole. While the diminished reactivity of the electron-poor alkynes was initially problematic, this feature was subsequently found to be pivotal in facilitating thermal control of mono- versus double 6-endo-trig ring-closure in the key step. Iodinated ullazine products were shown to undergo further facile transformation into more complex unsymmetrical targets. The synthetic strategies reported herein grant access to a host of symmetrically- and unsymmetrically-decorated fluorine-rich ullazines with potential value as light-harvesting materials (e.g., dye-sensitized solar cells, organic photovoltaics) or as advanced intermediates for further synthetic elaboration.
中文翻译:

通过控制环芳构化对称和不对称的富氟ullazines
我们报告了一系列的富氟ullazines通过高度电子缺乏炔烃的良好控制的环芳烃合成。具体地,通过高度电子贫的五氟磺酰化(即,含SF 5)的苯基乙炔基部分的受控亲电环化,由1-(2,6-二炔基苯基)-1 H-吡咯构建三个对称官能化和三个非对称官能化的脲嗪核心。到吡咯上。虽然最初缺乏电子的炔烃的反应性降低是有问题的,但随后发现此功能对于促进对单6-双内-trig和双6 -end-trig的热控制至关重要关键步骤中的闭环。碘化的ullazine产品被证明可以轻松地转化为更复杂的不对称靶标。本文报道的合成策略允许获得许多具有潜在价值的对称和不对称装饰的富氟ullazines作为光收集材料(例如染料敏化太阳能电池,有机光伏电池)或高级合成中间体,用于进一步的合成加工。
更新日期:2020-03-03
中文翻译:

通过控制环芳构化对称和不对称的富氟ullazines
我们报告了一系列的富氟ullazines通过高度电子缺乏炔烃的良好控制的环芳烃合成。具体地,通过高度电子贫的五氟磺酰化(即,含SF 5)的苯基乙炔基部分的受控亲电环化,由1-(2,6-二炔基苯基)-1 H-吡咯构建三个对称官能化和三个非对称官能化的脲嗪核心。到吡咯上。虽然最初缺乏电子的炔烃的反应性降低是有问题的,但随后发现此功能对于促进对单6-双内-trig和双6 -end-trig的热控制至关重要关键步骤中的闭环。碘化的ullazine产品被证明可以轻松地转化为更复杂的不对称靶标。本文报道的合成策略允许获得许多具有潜在价值的对称和不对称装饰的富氟ullazines作为光收集材料(例如染料敏化太阳能电池,有机光伏电池)或高级合成中间体,用于进一步的合成加工。











































 京公网安备 11010802027423号
京公网安备 11010802027423号