当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of highly functionalized 5,6-seco-grayanane diterpenoids as potent competitive PTP1B inhibitors
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020/02/04 , DOI: 10.1039/c9qo01538h Junfei Zhou 1, 2, 3, 4, 5 , Zhili Zuo 6, 7, 8, 9, 10 , Junjun Liu 1, 2, 3, 4, 5 , Hanqi Zhang 1, 2, 3, 4, 5 , Guijuan Zheng 1, 2, 3, 4, 5 , Guangmin Yao 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020/02/04 , DOI: 10.1039/c9qo01538h Junfei Zhou 1, 2, 3, 4, 5 , Zhili Zuo 6, 7, 8, 9, 10 , Junjun Liu 1, 2, 3, 4, 5 , Hanqi Zhang 1, 2, 3, 4, 5 , Guijuan Zheng 1, 2, 3, 4, 5 , Guangmin Yao 1, 2, 3, 4, 5
Affiliation
|
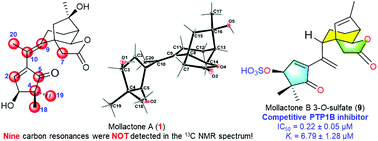
Protein tyrosine phosphatase 1B (PTP1B) is an important therapeutic target for type II diabetes mellitus. Mollactones A–C (1–3), three naturally occurring highly functionalized 5,6-seco-grayanane diterpenoids bearing a unique 3-oxa-tricyclo[4,3,2,02,6]undecane motif, and their plausible biosynthetic precursor rhodojaponin III (4) were isolated from Rhododendron molle. The 13C NMR spectrum of 1 exhibited only 11 carbon signals, instead of 20 carbon signals, and this challenging structure was finally defined by single-crystal X-ray diffraction analysis. This abnormal NMR phenomenon was elucidated by quantum chemical calculation. Mollactones A–C (1–3) showed significant PTP1B inhibitory activity in a competitive inhibition mode, and their structure–activity-relationships were investigated. Based on the molecular docking studies, mollactone B 3-O-sulfate (9) was rationally designed and prepared, and exhibited significant PTP1B inhibitory activity with an IC50 value of 0.22 ± 0.05 μM and a Ki value of 6.79 ± 1.28 μM. These results provide a basis to design novel competitive PTP1B inhibitors based on 5,6-seco-grayanane diterpenoids with 3-oxa-tricyclo[4,3,2,02,6]undecane and 5,5-dimethylcyclopent-2-en-1-one motifs.
中文翻译:

发现功能强大的5,6-seco-grayanane二萜作为有效的竞争性PTP1B抑制剂
蛋白酪氨酸磷酸酶1B(PTP1B)是II型糖尿病的重要治疗靶标。Mollactones A-C(1-3),三个天然存在的高度官能化的5,6-开环-grayanane二萜类化合物带有独特3-氧杂-三环[4,3,2,0 2,6 ]十一烷基序,和它们的合理的生物合成前体杜鹃皂苷III(4)是从杜鹃花中分离出来的。的13 C NMR图谱1仅显示出11个碳的信号,而不是20点碳的信号,并且这个挑战结构最后用单晶X射线衍射分析确定。通过量子化学计算可以阐明这种异常的NMR现象。甲内酯A–C(1–3)在竞争性抑制模式下显示出显着的PTP1B抑制活性,并研究了它们的结构-活性关系。基于分子对接研究,合理地设计和制备了内酯B 3- O-硫酸盐(9),并显示出显着的PTP1B抑制活性,IC 50值为0.22±0.05μM,K i值为6.79±1.28μM。这些结果提供了一个基础基于5,6-设计新颖竞争性抑制剂PTP1B山高-grayanane二萜类化合物与3-氧杂-三环[4,3,2,0 2,6 ]十一烷和5,5-二甲基环戊-2-烯-1-一个主题。
更新日期:2020-03-19
中文翻译:

发现功能强大的5,6-seco-grayanane二萜作为有效的竞争性PTP1B抑制剂
蛋白酪氨酸磷酸酶1B(PTP1B)是II型糖尿病的重要治疗靶标。Mollactones A-C(1-3),三个天然存在的高度官能化的5,6-开环-grayanane二萜类化合物带有独特3-氧杂-三环[4,3,2,0 2,6 ]十一烷基序,和它们的合理的生物合成前体杜鹃皂苷III(4)是从杜鹃花中分离出来的。的13 C NMR图谱1仅显示出11个碳的信号,而不是20点碳的信号,并且这个挑战结构最后用单晶X射线衍射分析确定。通过量子化学计算可以阐明这种异常的NMR现象。甲内酯A–C(1–3)在竞争性抑制模式下显示出显着的PTP1B抑制活性,并研究了它们的结构-活性关系。基于分子对接研究,合理地设计和制备了内酯B 3- O-硫酸盐(9),并显示出显着的PTP1B抑制活性,IC 50值为0.22±0.05μM,K i值为6.79±1.28μM。这些结果提供了一个基础基于5,6-设计新颖竞争性抑制剂PTP1B山高-grayanane二萜类化合物与3-氧杂-三环[4,3,2,0 2,6 ]十一烷和5,5-二甲基环戊-2-烯-1-一个主题。



























 京公网安备 11010802027423号
京公网安备 11010802027423号