当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 4-ethenyl quinazolines via rhodium(III)-catalyzed [5 + 1] annulation reaction of N-arylamidines with cyclopropenones
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/01/20 , DOI: 10.1039/c9qo01372e Huimin Xing 1, 2, 3, 4, 5 , Jian Chen 1, 2, 3, 4, 5 , Yuesen Shi 1, 2, 3, 4, 5 , Tianle Huang 1, 2, 3, 4, 5 , Li Hai 1, 2, 3, 4, 5 , Yong Wu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/01/20 , DOI: 10.1039/c9qo01372e Huimin Xing 1, 2, 3, 4, 5 , Jian Chen 1, 2, 3, 4, 5 , Yuesen Shi 1, 2, 3, 4, 5 , Tianle Huang 1, 2, 3, 4, 5 , Li Hai 1, 2, 3, 4, 5 , Yong Wu 1, 2, 3, 4, 5
Affiliation
|
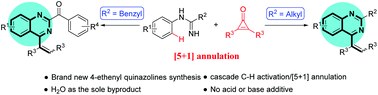
An unprecedented synthesis of 4-ethenyl quinazolines via a Rh(III)-catalyzed C–H activation and annulation reaction was described. In particular, when C-alkyl imidamides are benzyl groups, 2-benzoyl quinazolines can be obtained. This protocol enables the effective application of cyclopropenones, featuring high atom efficiency, broad substrate scope and mild reaction conditions.
中文翻译:

通过铑(III)催化的N-芳基idine与环丙烯酮的[5 +1]环合反应合成4-乙烯基喹唑啉
通过Rh(III)催化的C–H活化和环化反应,空前地合成了4-乙烯基喹唑啉。特别地,当C-烷基亚酰胺为苄基时,可获得2-苯甲酰基喹唑啉。该方案能够有效地应用环丙烯酮,具有高原子效率,广泛的底物范围和温和的反应条件。
更新日期:2020-02-18
中文翻译:

通过铑(III)催化的N-芳基idine与环丙烯酮的[5 +1]环合反应合成4-乙烯基喹唑啉
通过Rh(III)催化的C–H活化和环化反应,空前地合成了4-乙烯基喹唑啉。特别地,当C-烷基亚酰胺为苄基时,可获得2-苯甲酰基喹唑啉。该方案能够有效地应用环丙烯酮,具有高原子效率,广泛的底物范围和温和的反应条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号