当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bifunctional PtCu electrocatalysts for the N2 reduction reaction under ambient conditions and methanol oxidation
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020/02/07 , DOI: 10.1039/d0qi00035c Caihong Fang 1, 2, 3, 4, 5 , Jinwu Hu 1, 2, 3, 4, 5 , Xiaomin Jiang 1, 2, 3, 4, 5 , Zhiqing Cui 1, 2, 3, 4, 5 , Xiaoxiao Xu 1, 2, 3, 4, 5 , Ting Bi 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020/02/07 , DOI: 10.1039/d0qi00035c Caihong Fang 1, 2, 3, 4, 5 , Jinwu Hu 1, 2, 3, 4, 5 , Xiaomin Jiang 1, 2, 3, 4, 5 , Zhiqing Cui 1, 2, 3, 4, 5 , Xiaoxiao Xu 1, 2, 3, 4, 5 , Ting Bi 1, 2, 3, 4, 5
Affiliation
|
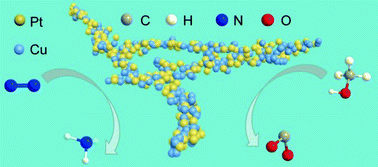
In our work, we demonstrated network PtCu nanocrystals, in which the tuneable composition, clean surface, and ultrasmall size (diameter < 5 nm) endow them with excellent electrocatalytic performances in both N2 reduction reaction under ambient conditions and methanol oxidation. A detailed investigation suggests that their electrocatalytic activity is highly composition dependent. We confirmed the production of NH3 from N2 through a systematic study. The optimum Pt6Cu nanoalloys exhibit 2.3-fold and 20.6-fold increase in reduction activity toward N2 compared with Pt and Cu nanocrystals. Their faradaic efficiency value was also highly improved. Moreover, these bifunctional Pt6Cu nanostructures also show excellent catalytic properties during methanol electrooxidation. The specific activity toward methanol oxidation reaches up to 21.3 mA cm−2, which is 3.6 times that of commercial Pt/C catalysts. This work thus provides a promising approach to design the alloy composition for bi- or multi-functional applications.
中文翻译:

用于环境条件下N2还原反应和甲醇氧化的双功能PtCu电催化剂
在我们的工作中,我们展示了网络PtCu纳米晶体,其中可调节的成分,清洁的表面和超小尺寸(直径<5 nm)赋予它们在环境条件下的N 2还原反应和甲醇氧化中均具有出色的电催化性能。详细的研究表明,它们的电催化活性高度依赖于组成。通过系统的研究,我们证实了N 2可以生成NH 3。与Pt和Cu纳米晶体相比,最佳的Pt 6 Cu纳米合金对N 2的还原活性提高了2.3倍和20.6倍。他们的法拉第效率值也大大提高了。此外,这些双功能铂6Cu纳米结构在甲醇电氧化过程中也显示出优异的催化性能。对甲醇氧化的比活性达到21.3mA cm -2,是市售Pt / C催化剂的3.6倍。因此,这项工作为设计用于双功能或多功能应用的合金成分提供了一种有前途的方法。
更新日期:2020-03-19
中文翻译:

用于环境条件下N2还原反应和甲醇氧化的双功能PtCu电催化剂
在我们的工作中,我们展示了网络PtCu纳米晶体,其中可调节的成分,清洁的表面和超小尺寸(直径<5 nm)赋予它们在环境条件下的N 2还原反应和甲醇氧化中均具有出色的电催化性能。详细的研究表明,它们的电催化活性高度依赖于组成。通过系统的研究,我们证实了N 2可以生成NH 3。与Pt和Cu纳米晶体相比,最佳的Pt 6 Cu纳米合金对N 2的还原活性提高了2.3倍和20.6倍。他们的法拉第效率值也大大提高了。此外,这些双功能铂6Cu纳米结构在甲醇电氧化过程中也显示出优异的催化性能。对甲醇氧化的比活性达到21.3mA cm -2,是市售Pt / C催化剂的3.6倍。因此,这项工作为设计用于双功能或多功能应用的合金成分提供了一种有前途的方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号