当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 and CH4 conversion in “real” gas mixtures in a gliding arc plasmatron: how do N2 and O2 affect the performance?
Green Chemistry ( IF 9.3 ) Pub Date : 2020/01/30 , DOI: 10.1039/c9gc03743h Joachim Slaets 1, 2, 3, 4, 5 , Maryam Aghaei 1, 2, 3, 4, 5 , Sara Ceulemans 1, 2, 3, 4, 5 , Senne Van Alphen 1, 2, 3, 4, 5 , Annemie Bogaerts 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020/01/30 , DOI: 10.1039/c9gc03743h Joachim Slaets 1, 2, 3, 4, 5 , Maryam Aghaei 1, 2, 3, 4, 5 , Sara Ceulemans 1, 2, 3, 4, 5 , Senne Van Alphen 1, 2, 3, 4, 5 , Annemie Bogaerts 1, 2, 3, 4, 5
Affiliation
|
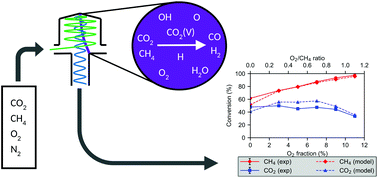
In this paper we study dry reforming of methane (DRM) in a gliding arc plasmatron (GAP) in the presence of N2 and O2. N2 is added to create a stable plasma at equal fractions of CO2 and CH4, and because emissions from industrial plants typically contain N2, while O2 is added to enhance the process. We test different gas mixing ratios to evaluate the conversion and energy cost. We obtain conversions between 31 and 52% for CO2 and between 55 and 99% for CH4, with total energy costs between 3.4 and 5.0 eV per molecule, depending on the gas mixture. This is very competitive when benchmarked with the literature. In addition, we present a chemical kinetics model to obtain deeper insight in the underlying plasma chemistry. This allows determination of the major reaction pathways to convert CO2 and CH4, in the presence of O2 and N2, into CO and H2. We show that N2 assists in the CO2 conversion, but part of the applied energy is also wasted in N2 excitation. Adding O2 enhances the CH4 conversion, and lowers the energy cost, while the CO2 conversion remains constant, and only slightly drops at the highest O2 fractions studied, when CH4 is fully oxidized into CO2.
中文翻译:

滑弧等离子加速器中“真实”气体混合物中的CO2和CH4转化:N2和O2如何影响性能?
在本文中,我们研究了在存在N 2和O 2的情况下,在滑弧等离子加速器(GAP)中对甲烷(DRM)进行干法重整。添加N 2以在等比例的CO 2和CH 4处产生稳定的等离子体,并且由于来自工厂的排放物通常包含N 2,而添加O 2则可以增强该过程。我们测试了不同的气体混合比,以评估转化率和能源成本。我们获得的CO 2转化率为31%至52 %,CH 4的转化率为55%至99%,根据混合气体的不同,每个分子的总能量成本在3.4至5.0 eV之间。当以文献为基准时,这是非常有竞争力的。此外,我们提出了一种化学动力学模型,以便在潜在的等离子体化学中获得更深入的了解。这允许确定在O 2和N 2存在下将CO 2和CH 4转化为CO和H 2的主要反应途径。我们表明,N 2有助于CO 2转化,但是在N 2激发中也浪费了一部分施加的能量。添加O 2可以提高CH 4的转化率,并降低能源成本,而CO当CH 4完全氧化为CO 2时, 2的转化率保持恒定,并且在研究的最高O 2分数下仅略有下降。
更新日期:2020-02-24
中文翻译:

滑弧等离子加速器中“真实”气体混合物中的CO2和CH4转化:N2和O2如何影响性能?
在本文中,我们研究了在存在N 2和O 2的情况下,在滑弧等离子加速器(GAP)中对甲烷(DRM)进行干法重整。添加N 2以在等比例的CO 2和CH 4处产生稳定的等离子体,并且由于来自工厂的排放物通常包含N 2,而添加O 2则可以增强该过程。我们测试了不同的气体混合比,以评估转化率和能源成本。我们获得的CO 2转化率为31%至52 %,CH 4的转化率为55%至99%,根据混合气体的不同,每个分子的总能量成本在3.4至5.0 eV之间。当以文献为基准时,这是非常有竞争力的。此外,我们提出了一种化学动力学模型,以便在潜在的等离子体化学中获得更深入的了解。这允许确定在O 2和N 2存在下将CO 2和CH 4转化为CO和H 2的主要反应途径。我们表明,N 2有助于CO 2转化,但是在N 2激发中也浪费了一部分施加的能量。添加O 2可以提高CH 4的转化率,并降低能源成本,而CO当CH 4完全氧化为CO 2时, 2的转化率保持恒定,并且在研究的最高O 2分数下仅略有下降。











































 京公网安备 11010802027423号
京公网安备 11010802027423号