当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical-induced regioselective C-3 thiomethylation of imidazopyridines via a three-component cross-coupling strategy
Green Chemistry ( IF 9.3 ) Pub Date : 2020/01/27 , DOI: 10.1039/c9gc04068d Jiangwei Wen 1, 2, 3, 4, 5 , Cong Niu 1, 2, 3, 4, 5 , Kelu Yan 1, 2, 3, 4, 5 , Xingda Cheng 1, 2, 3, 4, 5 , Ruike Gong 1, 2, 3, 4, 5 , Mengqian Li 1, 2, 3, 4, 5 , Yongqiang Guo 1, 2, 3, 4, 5 , Jianjing Yang 1, 2, 3, 4, 5 , Hua Wang 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020/01/27 , DOI: 10.1039/c9gc04068d Jiangwei Wen 1, 2, 3, 4, 5 , Cong Niu 1, 2, 3, 4, 5 , Kelu Yan 1, 2, 3, 4, 5 , Xingda Cheng 1, 2, 3, 4, 5 , Ruike Gong 1, 2, 3, 4, 5 , Mengqian Li 1, 2, 3, 4, 5 , Yongqiang Guo 1, 2, 3, 4, 5 , Jianjing Yang 1, 2, 3, 4, 5 , Hua Wang 1, 2, 3, 4, 5
Affiliation
|
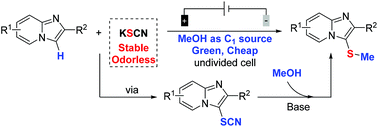
The electrochemical-induced regioselective C-3 thiomethylation of imidazopyridines has been initially accomplished via a three-component cross-coupling strategy using thiocyanate as the sulfur source and methanol as the methyl reagent. This protocol provides a green method for the thiomethylation of imidazopyridines without the need for any exogenous oxidants and metal catalysts under room temperature conditions. A wide range of functional groups were tolerated to produce regioselective C-3 thiomethylated products in high yields. Importantly, such an electrochemical-oxidation-induced thiomethylated reaction could be easily scaled up with excellent efficiency.
中文翻译:

通过三组分交叉偶联策略电化学诱导咪唑并吡啶类的区域选择性C-3硫甲基化
咪唑并吡啶类化合物的电化学诱导的区域选择性C-3硫代甲基化反应最初是通过三组分交叉偶联策略完成的,使用硫氰酸盐作为硫源,甲醇作为甲基试剂。该方案为咪唑并吡啶的硫代甲基化提供了一种绿色方法,在室温条件下无需任何外来氧化剂和金属催化剂。宽泛的官能团可以耐受,从而以高收率生产区域选择性的C-3硫代甲基化产品。重要的是,这种电化学氧化诱导的硫代甲基化反应可以容易地以优异的效率放大。
更新日期:2020-02-24
中文翻译:

通过三组分交叉偶联策略电化学诱导咪唑并吡啶类的区域选择性C-3硫甲基化
咪唑并吡啶类化合物的电化学诱导的区域选择性C-3硫代甲基化反应最初是通过三组分交叉偶联策略完成的,使用硫氰酸盐作为硫源,甲醇作为甲基试剂。该方案为咪唑并吡啶的硫代甲基化提供了一种绿色方法,在室温条件下无需任何外来氧化剂和金属催化剂。宽泛的官能团可以耐受,从而以高收率生产区域选择性的C-3硫代甲基化产品。重要的是,这种电化学氧化诱导的硫代甲基化反应可以容易地以优异的效率放大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号