当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial structure-governed SO2 resistance of Cu/TiO2 catalysts in the catalytic oxidation of CO
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020/01/29 , DOI: 10.1039/c9cy02405k Weiwei Yang 1, 2, 3, 4, 5 , Li Li 1, 2, 3, 4, 5 , Yarong Fang 1, 2, 3, 4, 5 , Yulong Shan 6, 7, 8, 9 , Jue Xu 1, 2, 3, 4, 5 , Huan Shen 1, 2, 3, 4, 5 , Yunbo Yu 6, 7, 8, 9 , Yanbing Guo 1, 2, 3, 4, 5 , Hong He 6, 7, 8, 9
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020/01/29 , DOI: 10.1039/c9cy02405k Weiwei Yang 1, 2, 3, 4, 5 , Li Li 1, 2, 3, 4, 5 , Yarong Fang 1, 2, 3, 4, 5 , Yulong Shan 6, 7, 8, 9 , Jue Xu 1, 2, 3, 4, 5 , Huan Shen 1, 2, 3, 4, 5 , Yunbo Yu 6, 7, 8, 9 , Yanbing Guo 1, 2, 3, 4, 5 , Hong He 6, 7, 8, 9
Affiliation
|
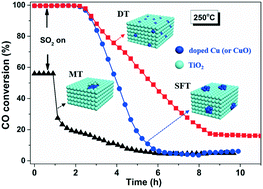
Three types of Cu–Ti interfacial structures have been fabricated by using different preparation methods. The mechanism regarding the interfacial effect on oxidation of CO and resistance to SO2 has been elaborated. It is proposed that an O vacancy-steered catalytic oxidation of CO prevails over an in situ Cu-doped TiO2 (DT) catalyst while a lattice O-initiated one proceeds on the other two catalysts that are obtained via solvent free and mechanical mixing methods. The former catalyst exhibits a strong Cu–Ti interface due to the high dispersion of copper species on TiO2 and partial incorporation of Cu2+ into the TiO2 matrix. This not only creates rich O vacancies, enhances O mobility and thus leads to superior redox ability of DT, hence promoting CO oxidation, but also restricts the interaction of SO2 with strongly-anchored Cu2+ species and thus increases the SO2 tolerance of DT. Meanwhile, the latter two catalysts have aggregated CuO either above or inside TiO2, which leads to rapid sulfation of CuO and deactivation of the catalysts.
中文翻译:

Cu / TiO2催化剂在CO催化氧化过程中界面控制的SO2耐受性
通过使用不同的制备方法已制备出三种类型的Cu-Ti界面结构。已经阐明了关于CO氧化的界面作用和抗SO 2的机理。建议在原位掺杂Cu的TiO 2(DT)催化剂上使用O空位控制的CO催化氧化,而通过无溶剂和机械混合方法获得的另两种催化剂则采用晶格O引发的催化剂进行。。前者的催化剂表现出很强的Cu-Ti界面,这是由于铜物种在TiO 2上的高度分散以及Cu 2+部分掺入TiO 2中矩阵。这不仅会产生丰富的O空位,增强O的迁移率,从而导致DT优异的氧化还原能力,从而促进CO氧化,而且限制了SO 2与强锚定Cu 2+物种的相互作用,从而提高了SO 2的耐受性。 DT。同时,后两种催化剂在TiO 2上方或内部聚集了CuO ,这导致CuO快速硫酸化和催化剂失活。
更新日期:2020-03-26
中文翻译:

Cu / TiO2催化剂在CO催化氧化过程中界面控制的SO2耐受性
通过使用不同的制备方法已制备出三种类型的Cu-Ti界面结构。已经阐明了关于CO氧化的界面作用和抗SO 2的机理。建议在原位掺杂Cu的TiO 2(DT)催化剂上使用O空位控制的CO催化氧化,而通过无溶剂和机械混合方法获得的另两种催化剂则采用晶格O引发的催化剂进行。。前者的催化剂表现出很强的Cu-Ti界面,这是由于铜物种在TiO 2上的高度分散以及Cu 2+部分掺入TiO 2中矩阵。这不仅会产生丰富的O空位,增强O的迁移率,从而导致DT优异的氧化还原能力,从而促进CO氧化,而且限制了SO 2与强锚定Cu 2+物种的相互作用,从而提高了SO 2的耐受性。 DT。同时,后两种催化剂在TiO 2上方或内部聚集了CuO ,这导致CuO快速硫酸化和催化剂失活。









































 京公网安备 11010802027423号
京公网安备 11010802027423号