当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How one-photon can induce water splitting into hydrogen peroxide and hydrogen by aluminum porphyrins. Rationale of the thermodynamics
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-01-23 , DOI: 10.1039/d0se00044b Fazalurahman Kuttassery 1, 2, 3, 4, 5 , Siby Mathew 1, 2, 3, 4, 5 , Hiroshi Tachibana 1, 2, 3, 4, 5 , Haruo Inoue 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-01-23 , DOI: 10.1039/d0se00044b Fazalurahman Kuttassery 1, 2, 3, 4, 5 , Siby Mathew 1, 2, 3, 4, 5 , Hiroshi Tachibana 1, 2, 3, 4, 5 , Haruo Inoue 1, 2, 3, 4, 5
Affiliation
|
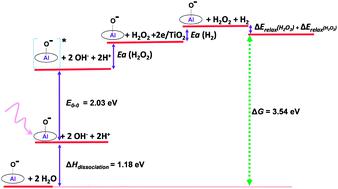
An alternative approach of two-electron water splitting into hydrogen peroxide and hydrogen by one-photon was developed to bypass the photon-flux density bottleneck in artificial photosynthesis catalysed by molecular catalysts. Here we report how one-photon can induce the water splitting by earth abundant aluminum porphyrins and how the thermodynamics is rationalized. A simple energy correlation between the input energy of one-photon excitation of aluminum porphyrin species (E00 = 2.03 eV) and output energy from the splitting of two water molecules into hydrogen peroxide and hydrogen (3.54 eV) reveals a requirement for additional energy from the surrounding heat bath to drive the catalytic cycle. We have estimated the activation energies for both the two-electron oxidation of water and hydrogen evolution by Arrhenius analysis in the temperature range of 295–346 K. The activation energy for the two-electron oxidation of water catalysed by AlTMPyP was found to be pH dependent (pH 7.0, 50.6 kJ mol−1 to pH 12.5, 26.5 kJ mol−1), which reflects that the reaction is driven by both water and hydroxide ions as the substrates. Under the pH 12.5 conditions the thermodynamic correlation was well rationalized by comparing the total energy input with the energy stored in the reaction forming H2O2 and H2.
中文翻译:

单光子如何诱导铝卟啉将水分解为过氧化氢和氢。热力学原理
提出了另一种通过单光子将两电子水分解为过氧化氢和氢的方法,以绕过分子催化剂催化的人工光合作用中的光子通量密度瓶颈。在这里,我们报告单光子如何通过富含地球的铝卟啉诱导水分裂,以及如何使热力学合理化。卟啉铝单光子激发的输入能量之间的简单能量相关(E 00= 2.03 eV)和两个水分子分裂成过氧化氢和氢的输出能量(3.54 eV)表明需要从周围的热浴中获得额外的能量来驱动催化循环。我们通过Arrhenius分析估算了在295-346 K的温度范围内水的双电子氧化和氢析出的活化能。发现AlTMPyP催化的水的双电子氧化的活化能为pH取决于(pH 7.0,50.6 kJ mol -1至pH 12.5,26.5 kJ mol -1),这表明反应是由水和氢氧根离子作为底物驱动的。在pH 12.5的条件下,通过比较输入的总能量与形成H 2 O 2和H 2的反应中存储的能量,可以使热力学相关性合理化。
更新日期:2020-01-23
中文翻译:

单光子如何诱导铝卟啉将水分解为过氧化氢和氢。热力学原理
提出了另一种通过单光子将两电子水分解为过氧化氢和氢的方法,以绕过分子催化剂催化的人工光合作用中的光子通量密度瓶颈。在这里,我们报告单光子如何通过富含地球的铝卟啉诱导水分裂,以及如何使热力学合理化。卟啉铝单光子激发的输入能量之间的简单能量相关(E 00= 2.03 eV)和两个水分子分裂成过氧化氢和氢的输出能量(3.54 eV)表明需要从周围的热浴中获得额外的能量来驱动催化循环。我们通过Arrhenius分析估算了在295-346 K的温度范围内水的双电子氧化和氢析出的活化能。发现AlTMPyP催化的水的双电子氧化的活化能为pH取决于(pH 7.0,50.6 kJ mol -1至pH 12.5,26.5 kJ mol -1),这表明反应是由水和氢氧根离子作为底物驱动的。在pH 12.5的条件下,通过比较输入的总能量与形成H 2 O 2和H 2的反应中存储的能量,可以使热力学相关性合理化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号