当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid CO2 capture-to-mineralisation in a scalable reactor
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020/01/21 , DOI: 10.1039/c9re00446g Ning Zhang 1, 1, 2, 3, 4 , Rafael M. Santos 1, 5, 6, 7 , Lidija Šiller 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020/01/21 , DOI: 10.1039/c9re00446g Ning Zhang 1, 1, 2, 3, 4 , Rafael M. Santos 1, 5, 6, 7 , Lidija Šiller 1, 2, 3, 4
Affiliation
|
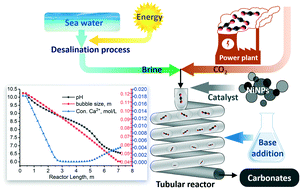
CO2 mineralisation is a process that can convert CO2 into solid carbonates for permanent storage. Our multiphase flow process uses an alkaline brine solution to capture gaseous CO2 and form carbonate particles in a continuous tubular reactor. In this research, a monoethanolamine (MEA) solution is utilised to synergistically boost CO2 solubility in the brine while neutralising the acidification caused by brine chloride ions left in the solution following the precipitation of alkaline earth metals. In this study, relatively low concentrations of MEA, ranging from 0.036 to 0.33 M, were investigated over a temperature range from 303 K to 323 K; these are significantly milder conditions than those used in traditional CO2 capture processes with MEA, which contributes to low energy demand of the process. Short residence time, in the order of a few minutes, is made possible by the high gas–liquid surface area for mass transfer and the rapid kinetics of aqueous phase carbonation reactions. Nickel nanoparticles (NiNPs) were tested as a catalytic additive to further accelerate the rate limiting step (CO2 dissolution) by accelerating the CO2 hydration reaction. Experimental results were used to develop and calibrate a one-dimensional time-dependent plug-flow model that incorporates transport and chemical speciation equations. The model is thus capable of predicting aqueous species and solid carbonate concentrations, fluid pressure and gas slug size as a function of reactor length. These in turn yield the carbonation conversion and total pressure drop, and provide mechanistic insight into the reactor processes that can be used for scale-up. The experimental and modelled results showed a good agreement for a wide range of conditions tested: effects of temperature, brine composition, MEA concentration, and gas–liquid flow ratio. Under optimum conditions, it was found that the reactor could achieve full conversion of calcium from the brine and CO2 from the gas phase, thus proving to be an efficient process with high atom economy.
中文翻译:

在可扩展的反应器中快速将二氧化碳捕集至矿化
CO 2矿化是一种可以将CO 2转化为固态碳酸盐以进行永久存储的过程。我们的多相流工艺使用碱性盐水溶液捕获气态CO 2并在连续管式反应器中形成碳酸盐颗粒。在这项研究中,单乙醇胺(MEA)溶液用于协同提高盐水中的CO 2溶解度,同时中和由碱土金属沉淀后溶液中残留的盐水氯化物离子引起的酸化作用。在这项研究中,在303 K至323 K的温度范围内研究了相对较低的MEA浓度,范围为0.036至0.33 M;与传统的CO 2相比,这些条件要温和得多使用MEA捕获过程,从而降低了过程的能源需求。高的气-液表面积用于传质以及水相碳酸化反应的快速动力学,使停留时间缩短至几分钟左右。测试了镍纳米颗粒(NiNPs)作为催化添加剂,以通过加速CO 2进一步加速限速步骤(CO 2溶解)水合反应。实验结果用于开发和校准一维时间相关的塞流模型,该模型结合了运输和化学形态方程。因此,该模型能够预测作为反应堆长度的函数的水质和固体碳酸盐浓度,流体压力和气团大小。这些继而产生碳酸化转化率和总压降,并为可用于规模放大的反应器过程提供机械原理。实验和模型化结果显示了在各种条件下的良好一致性:温度,盐水成分,MEA浓度和气液流量比的影响。在最佳条件下,发现反应器可以实现盐水和CO 2中钙的完全转化 从气相中提取,因此被证明是具有高原子经济性的有效方法。
更新日期:2020-03-03
中文翻译:

在可扩展的反应器中快速将二氧化碳捕集至矿化
CO 2矿化是一种可以将CO 2转化为固态碳酸盐以进行永久存储的过程。我们的多相流工艺使用碱性盐水溶液捕获气态CO 2并在连续管式反应器中形成碳酸盐颗粒。在这项研究中,单乙醇胺(MEA)溶液用于协同提高盐水中的CO 2溶解度,同时中和由碱土金属沉淀后溶液中残留的盐水氯化物离子引起的酸化作用。在这项研究中,在303 K至323 K的温度范围内研究了相对较低的MEA浓度,范围为0.036至0.33 M;与传统的CO 2相比,这些条件要温和得多使用MEA捕获过程,从而降低了过程的能源需求。高的气-液表面积用于传质以及水相碳酸化反应的快速动力学,使停留时间缩短至几分钟左右。测试了镍纳米颗粒(NiNPs)作为催化添加剂,以通过加速CO 2进一步加速限速步骤(CO 2溶解)水合反应。实验结果用于开发和校准一维时间相关的塞流模型,该模型结合了运输和化学形态方程。因此,该模型能够预测作为反应堆长度的函数的水质和固体碳酸盐浓度,流体压力和气团大小。这些继而产生碳酸化转化率和总压降,并为可用于规模放大的反应器过程提供机械原理。实验和模型化结果显示了在各种条件下的良好一致性:温度,盐水成分,MEA浓度和气液流量比的影响。在最佳条件下,发现反应器可以实现盐水和CO 2中钙的完全转化 从气相中提取,因此被证明是具有高原子经济性的有效方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号