当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cloning and expression of the flavin reductase LuxG from Photobacterium leiognathi YL and its improvement for NADH detection.
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-01-31 , DOI: 10.1039/c9pp00435a Guanhua Xuan 1 , Qilin Xiao , Jingxue Wang , Hong Lin
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-01-31 , DOI: 10.1039/c9pp00435a Guanhua Xuan 1 , Qilin Xiao , Jingxue Wang , Hong Lin
Affiliation
|
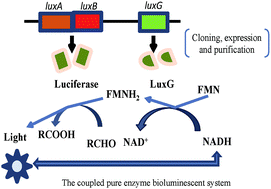
In the present study, we aimed to purify and characterize LuxG obtained from Photobacterium leiognathi YL and examine its improvement for NADH detection. To this end, we cloned and expressed the putative luxG gene of P. leiognathi YL in the Escherichia coli BL21 strain. The product of luxG is a flavin reductase that consists of 206 amino acids, corresponding to a subunit molecular mass of ∼26 kDa. Phylogenetic analysis demonstrated that P. leiognathi YL LuxG has a rather distant evolutionary relationship with Frase I of Aliivibrio fischeri and Frp of Vibrio harveyi, but a close evolutionary relationship with Fre from Escherichia coli, which are all enzymes related to oxido-reductase. Further comparison shows that the changes in the functionally conserved sites may contribute to the functional divergence of LuxG and Fre. LuxG could supply reduced flavin mononucleotide (FMN) for bacterial luminescence by catalyzing the oxidation of nicotinamide adenine dinucleotide hydrogen (NADH). Based on this, a coupled pure enzyme bioluminescent system was established and used for NADH detection. The NADH samples with concentrations of 0.1-1 nM were used to validate the linear relationship, and it was found that the logarithmic deviations were less than 3%, which showed more sensitive and stable results than the NADH detection by recombinant E. coli including the exogenously expressed luciferase and intrinsic Fre. Investigation of P. leiognathi YL LuxG would provide a basic understanding of its evolution, and structural and functional properties, which might contribute to the development of a NADH detection kit in the future.
中文翻译:

黄光杆菌YL的黄素还原酶LuxG的克隆表达及其对NADH检测的改进。
在本研究中,我们旨在纯化和鉴定得自Leiognathi YL细菌的LuxG,并研究其对NADH检测的改进。为此,我们在大肠杆菌BL21菌株中克隆并表达了假单胞菌YL的推定luxG基因。luxG的产物是黄素还原酶,由206个氨基酸组成,对应于约26 kDa的亚单位分子量。系统发育学分析表明,白僵菌YL LuxG与Aliivibrio fischeri的Frase I和哈维氏弧菌的Frp有很远的进化关系,但与大肠杆菌的Fre有着密切的进化关系,它们都是与氧化还原酶有关的酶。进一步的比较表明,功能保守位点的变化可能有助于LuxG和Fre的功能差异。LuxG可以通过催化烟酰胺腺嘌呤二核苷酸氢(NADH)的氧化来提供减少的黄素单核苷酸(FMN)用于细菌发光。基于此,建立了耦合的纯酶生物发光系统,并用于NADH检测。使用浓度为0.1-1 nM的NADH样品验证线性关系,发现对数偏差小于3%,这比重组E. coli(包括外源表达的萤光素酶和内在的Fre。对P. leiognathi YL LuxG的研究将提供对其进化以及结构和功能特性的基本了解,这可能有助于将来开发NADH检测试剂盒。
更新日期:2020-02-19
中文翻译:

黄光杆菌YL的黄素还原酶LuxG的克隆表达及其对NADH检测的改进。
在本研究中,我们旨在纯化和鉴定得自Leiognathi YL细菌的LuxG,并研究其对NADH检测的改进。为此,我们在大肠杆菌BL21菌株中克隆并表达了假单胞菌YL的推定luxG基因。luxG的产物是黄素还原酶,由206个氨基酸组成,对应于约26 kDa的亚单位分子量。系统发育学分析表明,白僵菌YL LuxG与Aliivibrio fischeri的Frase I和哈维氏弧菌的Frp有很远的进化关系,但与大肠杆菌的Fre有着密切的进化关系,它们都是与氧化还原酶有关的酶。进一步的比较表明,功能保守位点的变化可能有助于LuxG和Fre的功能差异。LuxG可以通过催化烟酰胺腺嘌呤二核苷酸氢(NADH)的氧化来提供减少的黄素单核苷酸(FMN)用于细菌发光。基于此,建立了耦合的纯酶生物发光系统,并用于NADH检测。使用浓度为0.1-1 nM的NADH样品验证线性关系,发现对数偏差小于3%,这比重组E. coli(包括外源表达的萤光素酶和内在的Fre。对P. leiognathi YL LuxG的研究将提供对其进化以及结构和功能特性的基本了解,这可能有助于将来开发NADH检测试剂盒。











































 京公网安备 11010802027423号
京公网安备 11010802027423号