当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-yield electrochemical hydrogen peroxide production from an enhanced two-electron oxygen reduction pathway by mesoporous nitrogen-doped carbon and manganese hybrid electrocatalysts.
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2020-02-12 , DOI: 10.1039/c9nh00783k Ayeong Byeon 1 , Jinwon Cho , Jong Min Kim , Keun Hwa Chae , Hee-Young Park , Seok Won Hong , Hyung Chul Ham , Seung Woo Lee , Ki Ro Yoon , Jin Young Kim
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2020-02-12 , DOI: 10.1039/c9nh00783k Ayeong Byeon 1 , Jinwon Cho , Jong Min Kim , Keun Hwa Chae , Hee-Young Park , Seok Won Hong , Hyung Chul Ham , Seung Woo Lee , Ki Ro Yoon , Jin Young Kim
Affiliation
|
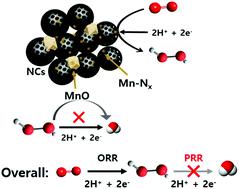
Electrochemical hydrogen peroxide (H2O2) production by the direct two-electron (2e-) oxygen reduction reaction (ORR) has received much attention as a promising alternative to the industrially developed anthraquinone fabrication process. Transition metal (M) and nitrogen doped carbon (M-N-C, M = Fe or Co) catalysts are known to be active for four electron ORR pathways via two + two electron transfer, where the former is for the ORR and the latter for the peroxide reduction reaction (PRR). Here, we report mesoporous N-doped carbon/manganese hybrid electrocatalysts composed of MnO and Mn-Nx coupled with N-doped carbons (Mn-O/N@NCs), which have led to the development of electrocatalysis towards the 2e- ORR route. Based on the structural and electrochemical characterization, the number of transferred electrons during the ORR on the Mn-O/N@NCs was found to be close to the theoretical value of the 2e- process, indicating their high activity toward H2O2. The favored ORR process arose due to the increased number of Mn-Nx sites within the mesoporous N-doped carbon materials. Furthermore, there was a strong indication that the PRR is significantly suppressed by adjacent MnO species, demonstrating its highly selective production of H2O2 (>80%) from the oxygen electrochemical process. The results of a real fuel cell device test demonstrated that an Mn-O/N@NC catalyst sustains a very stable current, and we attributed its outstanding activity to a combination of site-dependent facilitation of 2e- transfer and a favorable porosity for mass transport.
中文翻译:

介孔氮掺杂碳和锰杂化电催化剂通过增强的双电子氧还原途径生产高产率的电化学过氧化氢。
通过直接双电子(2e-)氧还原反应(ORR)生产电化学过氧化氢(H2O2)作为工业开发的蒽醌制备方法的一种有前途的替代方法已引起了广泛关注。已知过渡金属(M)和氮掺杂的碳(MNC,M = Fe或Co)催化剂通过两个+两个电子转移对四个电子ORR途径具有活性,其中前者用于ORR,后者用于过氧化物还原反应(PRR)。在这里,我们报道了由MnO和Mn-Nx与N掺杂的碳(Mn-O / N @ NCs)组成的介孔N掺杂的碳/锰杂化电催化剂,这导致了向2e-ORR途径的电催化的发展。 。根据结构和电化学特性,发现在Mn-O / N @ NCs的ORR过程中转移的电子数量接近2e-过程的理论值,表明它们对H2O2的活性很高。由于中孔氮掺杂碳材料中Mn-Nx位点数量的增加,出现了受人欢迎的ORR工艺。此外,有很强的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,并且我们将其出色的活性归因于2e转移的位置依赖性促进和良好的质量孔隙率的组合运输。表明它们对H2O2的高活性。由于中孔氮掺杂碳材料中Mn-Nx位点数量的增加,出现了受人欢迎的ORR工艺。此外,有很强的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,并且我们将其出色的活性归因于2e转移的位置依赖性促进和良好的质量孔隙率的组合运输。表明它们对H2O2的高活性。由于中孔掺氮碳材料中Mn-Nx位点数量的增加,出现了受人欢迎的ORR工艺。此外,有很强的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,我们将其出色的活性归因于2e-转移的位置依赖性促进和良好的质量孔隙率的组合运输。有一个强有力的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)的。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,我们将其出色的活性归因于2e-转移的位置依赖性促进和良好的质量孔隙率的组合运输。有一个强有力的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)的。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,并且我们将其出色的活性归因于2e转移的位置依赖性促进和良好的质量孔隙率的组合运输。
更新日期:2020-02-05
中文翻译:

介孔氮掺杂碳和锰杂化电催化剂通过增强的双电子氧还原途径生产高产率的电化学过氧化氢。
通过直接双电子(2e-)氧还原反应(ORR)生产电化学过氧化氢(H2O2)作为工业开发的蒽醌制备方法的一种有前途的替代方法已引起了广泛关注。已知过渡金属(M)和氮掺杂的碳(MNC,M = Fe或Co)催化剂通过两个+两个电子转移对四个电子ORR途径具有活性,其中前者用于ORR,后者用于过氧化物还原反应(PRR)。在这里,我们报道了由MnO和Mn-Nx与N掺杂的碳(Mn-O / N @ NCs)组成的介孔N掺杂的碳/锰杂化电催化剂,这导致了向2e-ORR途径的电催化的发展。 。根据结构和电化学特性,发现在Mn-O / N @ NCs的ORR过程中转移的电子数量接近2e-过程的理论值,表明它们对H2O2的活性很高。由于中孔氮掺杂碳材料中Mn-Nx位点数量的增加,出现了受人欢迎的ORR工艺。此外,有很强的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,并且我们将其出色的活性归因于2e转移的位置依赖性促进和良好的质量孔隙率的组合运输。表明它们对H2O2的高活性。由于中孔氮掺杂碳材料中Mn-Nx位点数量的增加,出现了受人欢迎的ORR工艺。此外,有很强的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,并且我们将其出色的活性归因于2e转移的位置依赖性促进和良好的质量孔隙率的组合运输。表明它们对H2O2的高活性。由于中孔掺氮碳材料中Mn-Nx位点数量的增加,出现了受人欢迎的ORR工艺。此外,有很强的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,我们将其出色的活性归因于2e-转移的位置依赖性促进和良好的质量孔隙率的组合运输。有一个强有力的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)的。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,我们将其出色的活性归因于2e-转移的位置依赖性促进和良好的质量孔隙率的组合运输。有一个强有力的迹象表明,相邻的MnO物种显着抑制了PRR,这表明它是通过氧电化学过程高度选择性地生成H2O2(> 80%)的。实际燃料电池装置测试的结果表明,Mn-O / N @ NC催化剂可维持非常稳定的电流,并且我们将其出色的活性归因于2e转移的位置依赖性促进和良好的质量孔隙率的组合运输。









































 京公网安备 11010802027423号
京公网安备 11010802027423号