当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting selectivity of paracellular pores for biomimetic applications
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2020-01-28 , DOI: 10.1039/c9me00177h Nandhini Rajagopal 1, 2, 3, 4 , Alejandro J. Durand 1, 2, 3, 4 , Shikha Nangia 1, 2, 3, 4
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2020-01-28 , DOI: 10.1039/c9me00177h Nandhini Rajagopal 1, 2, 3, 4 , Alejandro J. Durand 1, 2, 3, 4 , Shikha Nangia 1, 2, 3, 4
Affiliation
|
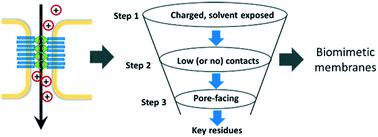
Biological systems exhibit diverse examples of controlled solute permeability and selectivity in cell and tissue barriers. The epithelial and endothelial cells lining each organ confer selectivity via tight junctions, physical fence-like structures that regulate paracellular transport, whose primary functional component is a claudin. Members of the claudin family of proteins undergo cis and trans assembly to control paracellular selectivity. However, based on the type of claudin and its expression level in a tissue, the tight junction selectivity varies from cationic to anionic or permeability changes from zero to leaky. In vitro and in vivo characterization of tight junction macroassemblies is a challenge, especially when molecular-level precision is essential for using nature's design principle for biomimetic applications, such as ion separation platforms and nanosensors. In the present work, we use a recently developed method, protein association energy landscape (PANEL), to exploit the cis architecture of claudin proteins to explain their paracellular selectivity. Using PANEL, we generated millions of claudin–claudin dimer geometries and analyzed the amino acid residue contacts. We demonstrate that a rigorous analysis of cis architectures can not only predict the critical residues responsible for tight junction selectivity, but the cis structures obtained can also provide putative tight junction pore configurations. A deeper understanding of tight junction architecture at a molecular level has been possible using specially designed computational tools and techniques. This approach has promise in determining the selectivity of tight junction proteins and their subsequent use in biomimetic ultrafiltration devices.
中文翻译:

预测用于仿生应用的细胞旁孔的选择性
生物系统展示了细胞和组织屏障中溶质渗透性和选择性可控的各种示例。沿每个器官排列的上皮细胞和内皮细胞通过紧密连接,调节副细胞转运的物理栅栏状结构赋予选择性,其主要功能成分是claudin。claudin蛋白家族的成员经过顺式和反式组装来控制细胞旁选择性。但是,基于claudin的类型及其在组织中的表达水平,紧密连接的选择性从阳离子到阴离子变化,或渗透性从零到渗漏变化。体外和体内紧密连接宏组件的特性表征是一个挑战,尤其是当分子级精度对于将自然界的设计原理用于仿生应用(例如离子分离平台和纳米传感器)至关重要时。在当前的工作中,我们使用一种最新开发的方法,蛋白质缔合能态图(PANEL),来开发claudin蛋白质的顺式结构来解释其旁细胞选择性。使用PANEL,我们生成了数百万个claudin-claudin二聚体几何结构,并分析了氨基酸残基接触。我们证明了对顺式结构的严格分析不仅可以预测负责紧密连接选择性的关键残基,还可以预测顺式获得的结构也可以提供假定的紧密连接孔构型。使用专门设计的计算工具和技术,可以在分子水平上更深入地了解紧密连接结构。这种方法在确定紧密连接蛋白的选择性及其在仿生超滤装置中的后续用途方面具有前景。
更新日期:2020-01-28
中文翻译:

预测用于仿生应用的细胞旁孔的选择性
生物系统展示了细胞和组织屏障中溶质渗透性和选择性可控的各种示例。沿每个器官排列的上皮细胞和内皮细胞通过紧密连接,调节副细胞转运的物理栅栏状结构赋予选择性,其主要功能成分是claudin。claudin蛋白家族的成员经过顺式和反式组装来控制细胞旁选择性。但是,基于claudin的类型及其在组织中的表达水平,紧密连接的选择性从阳离子到阴离子变化,或渗透性从零到渗漏变化。体外和体内紧密连接宏组件的特性表征是一个挑战,尤其是当分子级精度对于将自然界的设计原理用于仿生应用(例如离子分离平台和纳米传感器)至关重要时。在当前的工作中,我们使用一种最新开发的方法,蛋白质缔合能态图(PANEL),来开发claudin蛋白质的顺式结构来解释其旁细胞选择性。使用PANEL,我们生成了数百万个claudin-claudin二聚体几何结构,并分析了氨基酸残基接触。我们证明了对顺式结构的严格分析不仅可以预测负责紧密连接选择性的关键残基,还可以预测顺式获得的结构也可以提供假定的紧密连接孔构型。使用专门设计的计算工具和技术,可以在分子水平上更深入地了解紧密连接结构。这种方法在确定紧密连接蛋白的选择性及其在仿生超滤装置中的后续用途方面具有前景。









































 京公网安备 11010802027423号
京公网安备 11010802027423号