当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH dependence of the non-cooperative binding of Bi3+ to human apo-metallothionein 1A: kinetics, speciation, and stoichiometry.
Metallomics ( IF 2.9 ) Pub Date : 2020-02-07 , DOI: 10.1039/c9mt00285e Natalie C Korkola 1 , Patti M Scarrow , Martin J Stillman
Metallomics ( IF 2.9 ) Pub Date : 2020-02-07 , DOI: 10.1039/c9mt00285e Natalie C Korkola 1 , Patti M Scarrow , Martin J Stillman
Affiliation
|
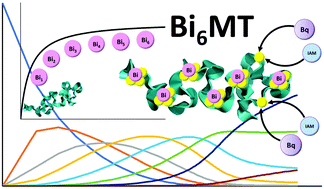
Bismuth is a well-known therapeutic agent that is used primarily for treatment against peptic ulcers. It has also had success in protecting against nephrotoxicity caused by the anticancer compound cisplatin by inducing the liver and kidney metalloprotein, metallothionein (MT) that then binds to the cisplatin. MT is a small, ubiquitous protein that binds monovalent, divalent, and trivalent metals using its abundant cysteine thiols (20 cysteines in the mammalian protein). It is important in the understanding of both these therapeutic applications to explore in detail the earliest stages of MT binding to bismuth salts. In this paper, we explored the binding of [Bi(cit)]- and [Bi(EDTA)]- to apo-MT 1a as the most basic of binding motifs. It was found that both Bi3+ salts bound in a non-cooperative stepwise manner to terminal cysteinal thiolates at pH 2.6, 5.0, and 7.4. We report that [Bi(EDTA)]- only binds stepwise up to Bi6MT, whereas [Bi(cit)]- forms up to Bi8MT, where the 7th and 8th Bi3+ appear to be adducts. Stepwise speciation analysis provided the 7 binding constants that decreased systematically from K1 to K7 indicating a non-cooperative binding profile. They are reported as log K1 = 27.89, log K2 = 27.78, log K3 = 27.77, log K4 = 27.62, log K5 = 27.32, log K6 = 26.75, and log K7 = 26.12, with log K[Bi(cit)]- determined to be 24.17. Cysteine modifications with benzoquinone and iodoacetamide revealed that when apoMT is fully metallated with Bi3+ there are two free cysteines, meaning 18 cysteines are used in binding the 6 Bi3+. Kinetic studies showed that [Bi(EDTA)]- binds very slowly at pH 2.6 (k = 0.0290 × 106 M-1 s-1) and approximately 2000 times faster at pH 7.4 (k = 66.5 × 106 M-1 s-1). [Bi(cit)]- binding at pH 2.6 was faster than [Bi(EDTA)]- (k = 672 × 106 M-1 s-1) at either pH level. The data strongly support a non-clustered binding motif, emphasizing the non-traditional pathway reported previously for As3+.
中文翻译:

Bi3 +与人类载脂蛋白硫蛋白1A非合作结合的pH依赖性:动力学,形态和化学计量。
铋是一种众所周知的治疗剂,主要用于治疗消化性溃疡。通过诱导肝脏和肾脏的金属蛋白,金属硫蛋白(MT)结合到顺铂上,它还成功地预防了由抗癌化合物顺铂引起的肾毒性。MT是一种小型无处不在的蛋白质,利用其丰富的半胱氨酸硫醇(哺乳动物蛋白质中的20个半胱氨酸)结合单价,二价和三价金属。在理解这两种治疗应用中,重要的是要详细探讨MT与铋盐结合的最早阶段。在本文中,我们探讨了[Bi(cit)]-和[Bi(EDTA)]-与apo-MT 1a的结合,这是最基本的结合基序。发现在pH 2下,两种Bi3 +盐均以非合作的逐步方式与末端半胱氨酸硫醇盐结合。6、5.0和7.4。我们报道[Bi(EDTA)]-仅逐步结合至Bi6MT,而[Bi(cit)]-形成至Bi8MT,其中第7个和第8个Bi3 +似乎是加合物。逐步的形态分析提供了从K1到K7逐渐降低的7个结合常数,这表明非合作结合谱。报告为log K1 = 27.89,log K2 = 27.78,log K3 = 27.77,log K4 = 27.62,log K5 = 27.32,log K6 = 26.75和log K7 = 26.12,其中log K [Bi(cit)]-确定为24.17。用苯醌和碘乙酰胺进行的半胱氨酸修饰显示,当apoMT与Bi3 +完全金属化时,有两个游离半胱氨酸,这意味着18个半胱氨酸用于结合6个Bi3 +。动力学研究表明[Bi(EDTA)]-在pH 2.6(k = 0.0290×106 M-1 s-1)时结合非常缓慢,在pH 7.4(k = 66)时快约2000倍。5×106 M-1 s-1)。在任何一个pH值下,[Bi(cit)]-在pH 2.6下的结合都比[Bi(EDTA)]-(k = 672×106 M-1 s-1)快。数据强烈支持非簇结合基序,强调了先前报道的As3 +的非传统途径。
更新日期:2020-03-26
中文翻译:

Bi3 +与人类载脂蛋白硫蛋白1A非合作结合的pH依赖性:动力学,形态和化学计量。
铋是一种众所周知的治疗剂,主要用于治疗消化性溃疡。通过诱导肝脏和肾脏的金属蛋白,金属硫蛋白(MT)结合到顺铂上,它还成功地预防了由抗癌化合物顺铂引起的肾毒性。MT是一种小型无处不在的蛋白质,利用其丰富的半胱氨酸硫醇(哺乳动物蛋白质中的20个半胱氨酸)结合单价,二价和三价金属。在理解这两种治疗应用中,重要的是要详细探讨MT与铋盐结合的最早阶段。在本文中,我们探讨了[Bi(cit)]-和[Bi(EDTA)]-与apo-MT 1a的结合,这是最基本的结合基序。发现在pH 2下,两种Bi3 +盐均以非合作的逐步方式与末端半胱氨酸硫醇盐结合。6、5.0和7.4。我们报道[Bi(EDTA)]-仅逐步结合至Bi6MT,而[Bi(cit)]-形成至Bi8MT,其中第7个和第8个Bi3 +似乎是加合物。逐步的形态分析提供了从K1到K7逐渐降低的7个结合常数,这表明非合作结合谱。报告为log K1 = 27.89,log K2 = 27.78,log K3 = 27.77,log K4 = 27.62,log K5 = 27.32,log K6 = 26.75和log K7 = 26.12,其中log K [Bi(cit)]-确定为24.17。用苯醌和碘乙酰胺进行的半胱氨酸修饰显示,当apoMT与Bi3 +完全金属化时,有两个游离半胱氨酸,这意味着18个半胱氨酸用于结合6个Bi3 +。动力学研究表明[Bi(EDTA)]-在pH 2.6(k = 0.0290×106 M-1 s-1)时结合非常缓慢,在pH 7.4(k = 66)时快约2000倍。5×106 M-1 s-1)。在任何一个pH值下,[Bi(cit)]-在pH 2.6下的结合都比[Bi(EDTA)]-(k = 672×106 M-1 s-1)快。数据强烈支持非簇结合基序,强调了先前报道的As3 +的非传统途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号