当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational design of graphitic carbon nitride photocatalysts for water splitting
Faraday Discussions ( IF 3.4 ) Pub Date : 2020-1-28 , DOI: 10.1039/c9fd00147f Gareth O. Hartley 1, 2, 3, 4, 5 , Natalia Martsinovich 1, 2, 3, 4
Faraday Discussions ( IF 3.4 ) Pub Date : 2020-1-28 , DOI: 10.1039/c9fd00147f Gareth O. Hartley 1, 2, 3, 4, 5 , Natalia Martsinovich 1, 2, 3, 4
Affiliation
|
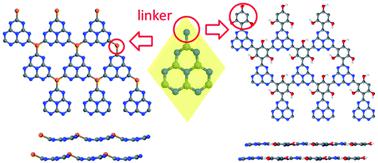
A series of structures based on graphitic carbon nitride (g-C3N4), a layered material composed of linked carbon–nitrogen heterocycles arranged in a plane, were investigated by density functional theory calculations. g-C3N4 is a semiconductor that absorbs UV light and visible light at the blue end of the visible spectrum, and is widely studied as a photocatalyst for water splitting; however, its photocatalytic efficiency is limited by its poor light-harvesting ability and low charge mobilities. Modifications to the g-C3N4 structure could greatly improve its optical and electronic properties and its photocatalytic efficiency. In this work, the g-C3N4 structure was modified by replacing the nitrogen linker with heteroatoms (phosphorus, boron) or aromatic groups (benzene, s-triazine and substituted benzenes). Two-dimensional (2D) sheets and three-dimensional (3D) multilayer structures with different stacking types were modelled. Several new structures were predicted to have electronic properties superior to g-C3N4 for use as water splitting photocatalysts. In particular, introduction of phosphorus, benzene and s-triazine groups led to band gaps smaller than in the standard g-C3N4 (down to 2.4 eV, corresponding to green light). Doping with boron in the linker positions dramatically reduced the band gap (to 1.6 eV, corresponding to red light); the doped material has the valence band position suitable for water oxidation. Our computational study shows that chemical modification of g-C3N4 is a powerful method to tune this material’s electronic properties and improve its photocatalytic activity.
中文翻译:

用于水分解的石墨氮化碳光催化剂的计算设计
通过密度泛函理论计算,研究了一系列基于石墨碳氮化物(gC 3 N 4)的结构,石墨是由排列在平面中的连接的碳氮杂环组成的层状材料。gC 3 N 4是在可见光谱的蓝色端吸收紫外光和可见光的半导体,并且被广泛用作光分解水的光催化剂。然而,其光催化效率受到光收集能力差和电荷迁移率低的限制。对gC 3 N 4结构的改性可以大大提高其光学和电子性能以及其光催化效率。在这项工作中,gC 3 N4结构是通过用杂原子(磷,硼)或芳族基团取代的氮连接体修饰(苯, š嗪和取代的苯)。对具有不同堆叠类型的二维(2D)薄片和三维(3D)多层结构进行了建模。预测有几种新结构具有比gC 3 N 4更好的电子性能,可用作水分解光催化剂。特别是,引入磷,苯和s-三嗪基团导致的带隙比标准gC 3 N 4小(低至2.4 eV,对应于绿灯)。在连接子位置掺杂硼会极大地减小带隙(至1.6 eV,对应于红光);掺杂材料的价带位置适合于水氧化。我们的计算研究表明,对gC 3 N 4进行化学修饰是调节该材料的电子性能并提高其光催化活性的有效方法。
更新日期:2020-01-28
中文翻译:

用于水分解的石墨氮化碳光催化剂的计算设计
通过密度泛函理论计算,研究了一系列基于石墨碳氮化物(gC 3 N 4)的结构,石墨是由排列在平面中的连接的碳氮杂环组成的层状材料。gC 3 N 4是在可见光谱的蓝色端吸收紫外光和可见光的半导体,并且被广泛用作光分解水的光催化剂。然而,其光催化效率受到光收集能力差和电荷迁移率低的限制。对gC 3 N 4结构的改性可以大大提高其光学和电子性能以及其光催化效率。在这项工作中,gC 3 N4结构是通过用杂原子(磷,硼)或芳族基团取代的氮连接体修饰(苯, š嗪和取代的苯)。对具有不同堆叠类型的二维(2D)薄片和三维(3D)多层结构进行了建模。预测有几种新结构具有比gC 3 N 4更好的电子性能,可用作水分解光催化剂。特别是,引入磷,苯和s-三嗪基团导致的带隙比标准gC 3 N 4小(低至2.4 eV,对应于绿灯)。在连接子位置掺杂硼会极大地减小带隙(至1.6 eV,对应于红光);掺杂材料的价带位置适合于水氧化。我们的计算研究表明,对gC 3 N 4进行化学修饰是调节该材料的电子性能并提高其光催化活性的有效方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号