当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combination of theoretical and in situ experimental investigations of the role of lithium dopant in manganese nitride: a two-stage reagent for ammonia synthesis
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-1-28 , DOI: 10.1039/c9fd00131j Said Laassiri 1, 2, 3, 4, 5 , Constantinos D. Zeinalipour-Yazdi 6, 7, 8, 9 , Nicolas Bion 2, 10, 11, 12, 13 , C. Richard A. Catlow 9, 14, 15, 16, 17 , Justin S. J. Hargreaves 9, 14, 18, 19, 20
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-1-28 , DOI: 10.1039/c9fd00131j Said Laassiri 1, 2, 3, 4, 5 , Constantinos D. Zeinalipour-Yazdi 6, 7, 8, 9 , Nicolas Bion 2, 10, 11, 12, 13 , C. Richard A. Catlow 9, 14, 15, 16, 17 , Justin S. J. Hargreaves 9, 14, 18, 19, 20
Affiliation
|
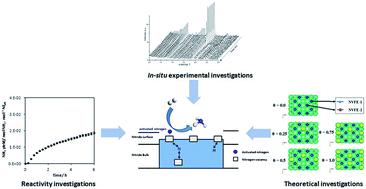
Manganese nitride related materials are of interest as two-stage reagents for ammonia synthesis via nitrogen chemical looping. However, unless they are doped with a co-cation, manganese nitrides are thermochemically stable and a high temperature is required to produce ammonia under reducing conditions, thereby hindering their use as nitrogen transfer materials. Nevertheless, when lithium is used as dopant, ammonia generation can be observed at a reaction temperature as low as 300 °C. In order to develop strategies for the improvement of the reactivity of nitride materials in the context of two-stage reagents, it is necessary to understand the intrinsic role of the dopant in the mechanism of ammonia synthesis. To this end, we have investigated the role of lithium in increasing the manganese nitride reactivity by in situ neutron diffraction studies and N2 and H2 isotopic exchange reactions supplemented by DFT calculations.
中文翻译:

锂掺杂剂在氮化锰中的作用的理论和原位实验研究相结合:氨合成的两阶段试剂
与氮化锰有关的材料作为通过氨合成氨的两阶段试剂而受到关注氮化学循环。但是,除非将它们掺有共阳离子,否则氮化锰是热化学稳定的,并且需要高温以在还原条件下产生氨,从而阻碍了它们用作氮转移材料。然而,当使用锂作为掺杂剂时,可以在低至300℃的反应温度下观察到氨的产生。为了在两阶段试剂的背景下制定提高氮化物材料反应性的策略,有必要了解掺杂剂在氨合成机理中的内在作用。为此,我们通过原位中子衍射研究以及N 2和H 2研究了锂在提高氮化锰反应性中的作用。 同位素交换反应辅以DFT计算。
更新日期:2020-01-28
中文翻译:

锂掺杂剂在氮化锰中的作用的理论和原位实验研究相结合:氨合成的两阶段试剂
与氮化锰有关的材料作为通过氨合成氨的两阶段试剂而受到关注氮化学循环。但是,除非将它们掺有共阳离子,否则氮化锰是热化学稳定的,并且需要高温以在还原条件下产生氨,从而阻碍了它们用作氮转移材料。然而,当使用锂作为掺杂剂时,可以在低至300℃的反应温度下观察到氨的产生。为了在两阶段试剂的背景下制定提高氮化物材料反应性的策略,有必要了解掺杂剂在氨合成机理中的内在作用。为此,我们通过原位中子衍射研究以及N 2和H 2研究了锂在提高氮化锰反应性中的作用。 同位素交换反应辅以DFT计算。











































 京公网安备 11010802027423号
京公网安备 11010802027423号