当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of pyrrole, imidazole, and pyrazole with ozone: kinetics and mechanisms
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-01-31 , DOI: 10.1039/c9ew01078e Agnes Tekle-Röttering 1, 2, 3, 4 , Sungeun Lim 5, 6, 7, 8, 9 , Erika Reisz 10, 11, 12, 13 , Holger V. Lutze 4, 14, 15, 16, 17 , Mohammad Sajjad Abdighahroudi 4, 14, 15, 16, 17 , Sarah Willach 4, 14, 15, 16, 17 , Winfried Schmidt 1, 2, 3, 4, 18 , Peter R. Tentscher 19, 20, 21, 22 , Daniel Rentsch 7, 8, 23, 24 , Christa S. McArdell 5, 6, 7, 8 , Torsten C. Schmidt 4, 14, 15, 16, 17 , Urs von Gunten 5, 6, 7, 8, 9
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2020-01-31 , DOI: 10.1039/c9ew01078e Agnes Tekle-Röttering 1, 2, 3, 4 , Sungeun Lim 5, 6, 7, 8, 9 , Erika Reisz 10, 11, 12, 13 , Holger V. Lutze 4, 14, 15, 16, 17 , Mohammad Sajjad Abdighahroudi 4, 14, 15, 16, 17 , Sarah Willach 4, 14, 15, 16, 17 , Winfried Schmidt 1, 2, 3, 4, 18 , Peter R. Tentscher 19, 20, 21, 22 , Daniel Rentsch 7, 8, 23, 24 , Christa S. McArdell 5, 6, 7, 8 , Torsten C. Schmidt 4, 14, 15, 16, 17 , Urs von Gunten 5, 6, 7, 8, 9
Affiliation
|
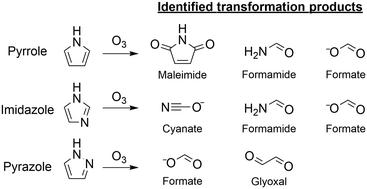
Five-membered nitrogen-containing heterocyclic compounds (azoles) belong to potential moieties in complex structures where transformations during ozonation can occur. This study focused on the azole–ozone chemistry of pyrrole, imidazole, and pyrazole as model compounds. Reaction kinetics and ozonation products were determined by kinetic and analytical methods including NMR, LC-HRMS/MS, HPLC-UV, and IC-MS. Analyses of reactive oxygen species (1O2, ˙OH, H2O2), quantum chemical computations (Gibbs energies), and kinetic simulations were used to further support the proposed reaction mechanisms. The species-specific second-order rate constants for the reactions of ozone with pyrrole and imidazole were (1.4 ± 1.1) × 106 M−1 s−1 and (2.3 ± 0.1) × 105 M−1 s−1, respectively. Pyrazole reacted more slowly with ozone at pH 7 (kapp = (5.6 ± 0.9) × 101 M−1 s−1). Maleimide was an identified product of pyrrole with a 34% yield. Together with other products, formate, formamide, and glyoxal, C and N mass balances of ∼50% were achieved. Imidazole reacted with ozone to cyanate, formamide, and formate (∼100% yields per transformed imidazole, respectively) with a closed mass balance. For pyrazole, a high ozone : pyrazole molar stoichiometry of 4.6 was found, suggesting that the transformation products contributed to the over-stoichiometric consumption of ozone (e.g., hydroxypyrazoles). Glyoxal and formate were the only identified transformation products (C mass balance of 65%). Overall, the identified major products are suspected to hydrolyze and/or be biodegraded and thereby abated by a biological post-treatment typically following ozonation. However, as substructures of more complex compounds (e.g., micropollutants), they might be more persistent during biological post-treatment.
中文翻译:

吡咯,咪唑和吡唑与臭氧的反应:动力学和机理
五元含氮杂环化合物(唑类)属于复杂结构中的潜在部分,在臭氧化过程中可能发生转化。这项研究集中于吡咯,咪唑和吡唑作为模型化合物的唑-臭氧化学反应。通过动力学和分析方法(包括NMR,LC-HRMS / MS,HPLC-UV和IC-MS)确定反应动力学和臭氧化产物。分析活性氧(1 O 2,˙OH,H 2 O 2),量子化学计算(吉布斯能)和动力学模拟可进一步支持所提出的反应机理。臭氧与吡咯和咪唑反应的物种特异性二阶速率常数为(1.4±1.1)×10 6M -1 s -1和(2.3±0.1)×10 5 M -1 s -1。吡唑与pH 7的臭氧反应更慢(k app =(5.6±0.9)×10 1 M -1 s -1)。马来酰亚胺是经鉴定的吡咯的产物,产率为34%。连同其他产品(甲酸盐,甲酰胺和乙二醛),碳和氮的质量平衡约为50%。咪唑与臭氧反应生成氰酸酯,甲酰胺和甲酸酯(每个转化的咪唑的收率分别为100%),并且质量平衡保持平衡。对于吡唑,发现较高的臭氧:吡唑摩尔化学计量比为4.6,这表明转化产物导致了化学计量比较高的臭氧消耗(例如,羟基吡唑)。乙二醛和甲酸是唯一鉴定出的转化产物(C质量平衡为65%)。总体而言,怀疑所鉴定的主要产品会水解和/或被生物降解,从而通常在臭氧化之后通过生物学后处理而减弱。但是,作为更复杂的化合物(例如,微污染物)的亚结构,它们在生物后处理过程中可能会更持久。
更新日期:2020-01-31
中文翻译:

吡咯,咪唑和吡唑与臭氧的反应:动力学和机理
五元含氮杂环化合物(唑类)属于复杂结构中的潜在部分,在臭氧化过程中可能发生转化。这项研究集中于吡咯,咪唑和吡唑作为模型化合物的唑-臭氧化学反应。通过动力学和分析方法(包括NMR,LC-HRMS / MS,HPLC-UV和IC-MS)确定反应动力学和臭氧化产物。分析活性氧(1 O 2,˙OH,H 2 O 2),量子化学计算(吉布斯能)和动力学模拟可进一步支持所提出的反应机理。臭氧与吡咯和咪唑反应的物种特异性二阶速率常数为(1.4±1.1)×10 6M -1 s -1和(2.3±0.1)×10 5 M -1 s -1。吡唑与pH 7的臭氧反应更慢(k app =(5.6±0.9)×10 1 M -1 s -1)。马来酰亚胺是经鉴定的吡咯的产物,产率为34%。连同其他产品(甲酸盐,甲酰胺和乙二醛),碳和氮的质量平衡约为50%。咪唑与臭氧反应生成氰酸酯,甲酰胺和甲酸酯(每个转化的咪唑的收率分别为100%),并且质量平衡保持平衡。对于吡唑,发现较高的臭氧:吡唑摩尔化学计量比为4.6,这表明转化产物导致了化学计量比较高的臭氧消耗(例如,羟基吡唑)。乙二醛和甲酸是唯一鉴定出的转化产物(C质量平衡为65%)。总体而言,怀疑所鉴定的主要产品会水解和/或被生物降解,从而通常在臭氧化之后通过生物学后处理而减弱。但是,作为更复杂的化合物(例如,微污染物)的亚结构,它们在生物后处理过程中可能会更持久。











































 京公网安备 11010802027423号
京公网安备 11010802027423号