当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Incorporation of Pb(II) into hematite during ferrihydrite transformation
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020/01/28 , DOI: 10.1039/c9en01355e Yang Lu 1, 2, 3, 4, 5 , Shiwen Hu 1, 2, 3, 4, 5 , Zheng Liang 5, 6, 7 , Mengqiang Zhu 8, 9, 10, 11 , Zimeng Wang 7, 12, 13, 14 , Xiaoming Wang 15, 16, 17, 18, 19 , Yuzhen Liang 1, 2, 3, 4, 5 , Zhi Dang 1, 2, 3, 4, 5 , Zhenqing Shi 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020/01/28 , DOI: 10.1039/c9en01355e Yang Lu 1, 2, 3, 4, 5 , Shiwen Hu 1, 2, 3, 4, 5 , Zheng Liang 5, 6, 7 , Mengqiang Zhu 8, 9, 10, 11 , Zimeng Wang 7, 12, 13, 14 , Xiaoming Wang 15, 16, 17, 18, 19 , Yuzhen Liang 1, 2, 3, 4, 5 , Zhi Dang 1, 2, 3, 4, 5 , Zhenqing Shi 1, 2, 3, 4, 5
Affiliation
|
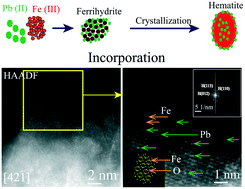
Ferrihydrite is ubiquitous in natural environments, and its transformation significantly influences the fate of heavy metals. Although Pb(II) adsorption on iron oxides has been extensively studied, there is still a knowledge gap on the fate of Pb during the dynamic processes of iron oxide transformation. In this study, a set of wet chemistry experiments, spherical aberration corrected scanning transmission electron microscopy (Cs-STEM) integrated with energy dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), and X-ray absorption spectroscopy (XAS) were used to unravel Pb interactions with iron oxides during ferrihydrite transformation processes under abiotic conditions. Wet chemistry experiments, STEM-EDS elemental mapping and line scans, and quantitative analysis suggested that Pb penetrated into hematite nanoparticles during the transformation processes. STEM analysis at sub-nanoscales, XRD fine scans, and XAS analysis provided evidence of Pb incorporation into the crystal structures of hematite nanoparticles, in which Pb distributed along the zone axis and enlarged the lattice distance of hematite. These results advanced our understanding of the dynamic interactions of Pb with iron oxides by offering new perspectives about the critical roles of chemical speciation, nano-scale spatial distribution, and atomic coordination environments in controlling the geochemical dynamics of heavy metals.
中文翻译:

水铁矿转变过程中将Pb(II)掺入赤铁矿
水铁矿在自然环境中无处不在,其转变显着影响重金属的命运。虽然铅(II)在氧化铁上的吸附已被广泛研究,在氧化铁转变的动态过程中,铅的命运仍然存在知识鸿沟。在这项研究中,进行了一组湿化学实验,结合了能量色散X射线光谱(EDS),X射线衍射(XRD)和X射线吸收光谱的球差校正扫描透射电子显微镜(Cs-STEM)( XAS)被用来揭示非生物条件下水铁矿转化过程中Pb与氧化铁的相互作用。湿化学实验,STEM-EDS元素映射和线扫描以及定量分析表明,Pb在转化过程中渗透到赤铁矿纳米颗粒中。亚纳米级的STEM分析,XRD精细扫描,XAS分析提供了Pb掺入赤铁矿纳米颗粒晶体结构的证据,其中Pb沿区域轴分布并扩大了赤铁矿的晶格距离。这些结果通过提供有关化学形态,纳米级空间分布和原子配位环境在控制重金属地球化学动力学中的关键作用的新观点,提高了我们对Pb与氧化铁动力学相互作用的理解。
更新日期:2020-03-21
中文翻译:

水铁矿转变过程中将Pb(II)掺入赤铁矿
水铁矿在自然环境中无处不在,其转变显着影响重金属的命运。虽然铅(II)在氧化铁上的吸附已被广泛研究,在氧化铁转变的动态过程中,铅的命运仍然存在知识鸿沟。在这项研究中,进行了一组湿化学实验,结合了能量色散X射线光谱(EDS),X射线衍射(XRD)和X射线吸收光谱的球差校正扫描透射电子显微镜(Cs-STEM)( XAS)被用来揭示非生物条件下水铁矿转化过程中Pb与氧化铁的相互作用。湿化学实验,STEM-EDS元素映射和线扫描以及定量分析表明,Pb在转化过程中渗透到赤铁矿纳米颗粒中。亚纳米级的STEM分析,XRD精细扫描,XAS分析提供了Pb掺入赤铁矿纳米颗粒晶体结构的证据,其中Pb沿区域轴分布并扩大了赤铁矿的晶格距离。这些结果通过提供有关化学形态,纳米级空间分布和原子配位环境在控制重金属地球化学动力学中的关键作用的新观点,提高了我们对Pb与氧化铁动力学相互作用的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号