当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A robust hybrid nanozyme@hydrogel platform as a biomimetic cascade bioreactor for combination antitumor therapy.
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-01-28 , DOI: 10.1039/c9bm01837a Yijun Hao 1 , Yandi Liu 1 , Yingjiao Wu 1 , Na Tao 1 , Dongyang Lou 1 , Juan Li 1 , Xiaoyi Sun 1 , You-Nian Liu 1
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-01-28 , DOI: 10.1039/c9bm01837a Yijun Hao 1 , Yandi Liu 1 , Yingjiao Wu 1 , Na Tao 1 , Dongyang Lou 1 , Juan Li 1 , Xiaoyi Sun 1 , You-Nian Liu 1
Affiliation
|
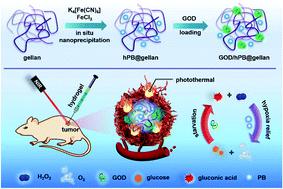
The development of highly effective and minimally invasive approaches for cancer treatment is the ultimate goal. Herein, an injectable hybrid hydrogel as a biomimetic cascade bioreactor is designed for combination antitumor therapy by providing spatiotemporally-controlled and long-term delivery of therapeutic agents. This hybrid nanozyme@hydrogel (hPB@gellan) is doped with Prussian blue (PB) nanoparticles via the in situ nanoprecipitation method in the polysaccharide gellan matrix. The obtained PB nanoparticles have a small size of 10 nm and play dual roles as a photothermal agent with a photothermal conversion efficiency of 59.6% and as a nanozyme to decompose hydrogen peroxide into oxygen. By incorporating glucose oxidase (GOD) into the hybrid hydrogel, a cascade bioreactor is formed for PB-promoted glucose consumption. Owing to its shear-thinning and self-recovery properties, the hybrid hydrogel is locally administered into tumors, and shows long-term resistance against body clearance and metabolism. The in vivo antitumor results demonstrate that the tumors in the group of combined photothermal and starvation therapy (GOD/hPB@gellan + NIR) are greatly eliminated with a tumor suppression rate of 99.7% 22 days after the treatment. The outstanding antitumor performance is attributed to the main attack by NIR-triggered hyperthermia and the holding attack by GOD-mediated starvation from the catalytic bioreactor of the hybrid hydrogel. Taking into consideration the advantages of biosafety, simple synthetic approaches and facile manipulation in treatment, the hybrid hydrogel has great potential for clinical translation.
中文翻译:

强大的杂合纳米酶@水凝胶平台,可作为仿生级联生物反应器用于联合抗肿瘤治疗。
最终目标是开发高效,微创的癌症治疗方法。本文中,通过提供时空控制和长期递送治疗剂,设计了作为仿生级联生物反应器的可注射杂化水凝胶,用于组合抗肿瘤治疗。该杂合纳米酶@水凝胶(hPB @ gellan)通过原位纳米沉淀法在多糖结冷基质中掺杂有普鲁士蓝(PB)纳米颗粒。所获得的PB纳米颗粒具有10nm的小尺寸,并且具有光热转化效率为59.6%的光热剂和将过氧化氢分解成氧的纳米酶的双重作用。通过将葡萄糖氧化酶(GOD)掺入杂化水凝胶中,形成了级联生物反应器,用于PB促进的葡萄糖消耗。由于其剪切变稀和自我恢复的特性,该混合水凝胶可局部施用于肿瘤,并显示出对机体清除和新陈代谢的长期抵抗力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。混合水凝胶可局部施用于肿瘤,并显示出对身体清除和新陈代谢的长期抵抗力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。混合水凝胶可局部施用于肿瘤,并显示出对身体清除和新陈代谢的长期抵抗力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。
更新日期:2020-01-28
中文翻译:

强大的杂合纳米酶@水凝胶平台,可作为仿生级联生物反应器用于联合抗肿瘤治疗。
最终目标是开发高效,微创的癌症治疗方法。本文中,通过提供时空控制和长期递送治疗剂,设计了作为仿生级联生物反应器的可注射杂化水凝胶,用于组合抗肿瘤治疗。该杂合纳米酶@水凝胶(hPB @ gellan)通过原位纳米沉淀法在多糖结冷基质中掺杂有普鲁士蓝(PB)纳米颗粒。所获得的PB纳米颗粒具有10nm的小尺寸,并且具有光热转化效率为59.6%的光热剂和将过氧化氢分解成氧的纳米酶的双重作用。通过将葡萄糖氧化酶(GOD)掺入杂化水凝胶中,形成了级联生物反应器,用于PB促进的葡萄糖消耗。由于其剪切变稀和自我恢复的特性,该混合水凝胶可局部施用于肿瘤,并显示出对机体清除和新陈代谢的长期抵抗力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。混合水凝胶可局部施用于肿瘤,并显示出对身体清除和新陈代谢的长期抵抗力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。混合水凝胶可局部施用于肿瘤,并显示出对身体清除和新陈代谢的长期抵抗力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。体内抗肿瘤结果表明,光热和饥饿联合疗法(GOD / hPB @ gellan + NIR)治疗组中的肿瘤在治疗后22天的抑制率为99.7%。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。出色的抗肿瘤性能归因于NIR触发的热疗的主要攻击以及来自混合水凝胶催化生物反应器的GOD介导的饥饿的保持攻击。考虑到生物安全性,简单的合成方法和操作简便的优势,混合水凝胶在临床翻译中具有巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号