当前位置:
X-MOL 学术
›
Process Saf. Environ. Prot.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Employing sodium hydroxide in desulfurization of the actual heavy crude oil: Theoretical optimization and experimental evaluation
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.psep.2020.01.036 Yusra A. Abd Al-Khodor , Talib M. Albayati
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.psep.2020.01.036 Yusra A. Abd Al-Khodor , Talib M. Albayati
|
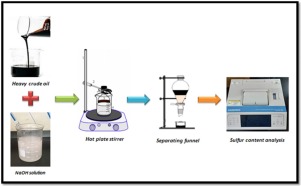
Abstract The desulfurization of the actual heavy crude oil is one of the most important processes in petroleum industries due to the low quality of those types of oil and containing large amounts of sulfur compounds, high viscosity and density. In the present work, the desulfurization of the actual heavy crude oil with a sulfur content 5.8 wt. % from Al-Halfaya Oil Field in southern Iraq was studied using a sodium hydroxide-assisted process. Effects of the operating conditions such as: reaction time (30–60 min), temperature (30–50 °C), the amount of NaOH in its solution (10–30 gm), and mixing speed (300–500 rpm) were investigated. The desulfurization process was achieved in a batch reactor by implementation of the experimental design technique. The objective function (response) was the sulfur content wt. % while a response surface method (RSM) was applied to define the significant factors that affect the desulfurization process. It was found that effects of the four variables take the following sequence: mixing speed > weight of NaOH > time > temperature. The optimum conditions of the proposed model were obtained using optimization techniques and found as follows: time = 60 min., temperature = 40 °C, NaOH solution = 18gm and mixing speed = 500 rpm. The optimum conditions of the sulfur content were applied experimentally and theoretically was equal to 2.5 and 2.3 wt. %, respectively. It is concluded that the efficiency of the sulfur removal content for actual heavy crude oil by this process was 56.89 %.
中文翻译:

氢氧化钠在实际重质原油脱硫中的应用:理论优化与实验评价
摘要 重质原油的脱硫是石油工业中最重要的工艺之一,因为重质原油质量低,含硫量大,粘度和密度大。在目前的工作中,对含硫量为 5.8 wt. 的实际重质原油进行脱硫。使用氢氧化钠辅助工艺研究了伊拉克南部 Al-Halfaya 油田的 %。操作条件的影响,例如:反应时间(30-60 分钟)、温度(30-50 °C)、溶液中 NaOH 的量(10-30 gm)和混合速度(300-500 rpm)是调查。通过实施实验设计技术,在间歇反应器中实现了脱硫过程。目标函数(响应)是硫含量wt。% 而应用响应面法 (RSM) 来定义影响脱硫过程的重要因素。发现四个变量的影响顺序如下:混合速度> NaOH 重量> 时间> 温度。所提出模型的最佳条件是使用优化技术获得的,结果如下:时间 = 60 分钟,温度 = 40 °C,NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。发现四个变量的影响顺序如下:混合速度> NaOH 重量> 时间> 温度。所提出模型的最佳条件是使用优化技术获得的,结果如下:时间 = 60 分钟,温度 = 40 °C,NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。发现四个变量的影响顺序如下:混合速度> NaOH 重量> 时间> 温度。所提出模型的最佳条件是使用优化技术获得的,结果如下:时间 = 60 分钟,温度 = 40 °C,NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。
更新日期:2020-04-01
中文翻译:

氢氧化钠在实际重质原油脱硫中的应用:理论优化与实验评价
摘要 重质原油的脱硫是石油工业中最重要的工艺之一,因为重质原油质量低,含硫量大,粘度和密度大。在目前的工作中,对含硫量为 5.8 wt. 的实际重质原油进行脱硫。使用氢氧化钠辅助工艺研究了伊拉克南部 Al-Halfaya 油田的 %。操作条件的影响,例如:反应时间(30-60 分钟)、温度(30-50 °C)、溶液中 NaOH 的量(10-30 gm)和混合速度(300-500 rpm)是调查。通过实施实验设计技术,在间歇反应器中实现了脱硫过程。目标函数(响应)是硫含量wt。% 而应用响应面法 (RSM) 来定义影响脱硫过程的重要因素。发现四个变量的影响顺序如下:混合速度> NaOH 重量> 时间> 温度。所提出模型的最佳条件是使用优化技术获得的,结果如下:时间 = 60 分钟,温度 = 40 °C,NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。发现四个变量的影响顺序如下:混合速度> NaOH 重量> 时间> 温度。所提出模型的最佳条件是使用优化技术获得的,结果如下:时间 = 60 分钟,温度 = 40 °C,NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。发现四个变量的影响顺序如下:混合速度> NaOH 重量> 时间> 温度。所提出模型的最佳条件是使用优化技术获得的,结果如下:时间 = 60 分钟,温度 = 40 °C,NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。NaOH 溶液 = 18gm,混合速度 = 500 rpm。硫含量的最佳条件是通过实验应用的,理论上等于 2.5 和 2.3 重量。%, 分别。结果表明,该工艺对实际重质原油的脱硫效率为56.89%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号