当前位置:
X-MOL 学术
›
Chem. Geol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The role of clay surfaces in the heterogeneous nucleation of calcite: Molecular dynamics simulations of cluster formation and attachment
Chemical Geology ( IF 3.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.chemgeo.2020.119497 Melinda A. Fodor , Zoltán Ható , Tamás Kristóf , Mihály Pósfai
Chemical Geology ( IF 3.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.chemgeo.2020.119497 Melinda A. Fodor , Zoltán Ható , Tamás Kristóf , Mihály Pósfai
|
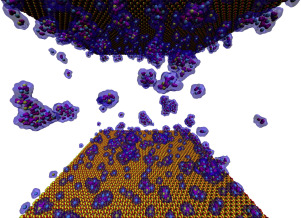
Abstract In many environments calcium carbonate minerals precipitate in the presence of clay minerals, and observations suggest that clays, particularly smectites, facilitate carbonate formation. In order to understand the interactions between clay surfaces and carbonate-precipitating solutions, we built model aqueous solutions of various compositions (containing Ca2+, Mg2+ and CO32– ions) between layers of clay minerals (montmorillonite and kaolinite), and performed extensive molecular dynamics simulations. The results were compared with simulations for bulk solutions. Contrary to intuition, ionic clusters formed preferentially in the interlayer solution (instead of on the clay surface). The clusters grew both by the association of individual ions and aggregation, and were adsorbed to the clay surfaces with distinctly different efficiencies in the various systems. Montmorillonite was found to be more efficient than kaolinite in capturing clusters from solution. However, the efficiency of anchoring ionic clusters to the clay surfaces strongly depended on the Na+ concentration of the solution, since Na+ appeared to strongly attach to the surface and thereby block it from clusters in the solution. Montmorillonite (and probably other smectite clays as well) may thus have an important role in certain, primarily freshwater, systems in the localization of ionic clusters on its surface, thereby promoting the nucleation and templated growth of crystalline calcium‑magnesium carbonate minerals.
中文翻译:

粘土表面在方解石异质成核中的作用:簇形成和附着的分子动力学模拟
摘要 在许多环境中,碳酸钙矿物在粘土矿物存在下沉淀,观察表明粘土,尤其是蒙脱石,促进碳酸盐的形成。为了了解粘土表面和碳酸盐沉淀溶液之间的相互作用,我们在粘土矿物(蒙脱石和高岭石)层之间建立了各种成分(包含 Ca2+、Mg2+ 和 CO32- 离子)的模型水溶液,并进行了广泛的分子动力学模拟. 将结果与批量解决方案的模拟进行比较。与直觉相反,离子簇优先在夹层溶液中形成(而不是在粘土表面)。簇通过单个离子的结合和聚集而增长,并在不同系统中以明显不同的效率吸附到粘土表面。发现蒙脱石在从溶液中捕获簇方面比高岭石更有效。然而,将离子簇锚定到粘土表面的效率在很大程度上取决于溶液的 Na+ 浓度,因为 Na+ 似乎强烈附着在表面上,从而阻止它与溶液中的簇连接。因此,蒙脱石(可能还有其他绿土粘土)可能在某些主要是淡水的系统中发挥重要作用,将离子簇定位在其表面,从而促进结晶碳酸钙镁矿物的成核和模板化生长。将离子簇固定到粘土表面的效率很大程度上取决于溶液的 Na+ 浓度,因为 Na+ 似乎强烈附着在表面上,从而阻止它与溶液中的簇接触。因此,蒙脱石(可能还有其他绿土粘土)可能在某些主要是淡水的系统中发挥重要作用,将离子簇定位在其表面,从而促进结晶碳酸钙镁矿物的成核和模板化生长。将离子簇固定到粘土表面的效率很大程度上取决于溶液的 Na+ 浓度,因为 Na+ 似乎强烈附着在表面上,从而阻止它与溶液中的簇接触。因此,蒙脱石(可能还有其他绿土粘土)可能在某些主要是淡水的系统中发挥重要作用,将离子簇定位在其表面,从而促进结晶碳酸钙镁矿物的成核和模板化生长。
更新日期:2020-04-01
中文翻译:

粘土表面在方解石异质成核中的作用:簇形成和附着的分子动力学模拟
摘要 在许多环境中,碳酸钙矿物在粘土矿物存在下沉淀,观察表明粘土,尤其是蒙脱石,促进碳酸盐的形成。为了了解粘土表面和碳酸盐沉淀溶液之间的相互作用,我们在粘土矿物(蒙脱石和高岭石)层之间建立了各种成分(包含 Ca2+、Mg2+ 和 CO32- 离子)的模型水溶液,并进行了广泛的分子动力学模拟. 将结果与批量解决方案的模拟进行比较。与直觉相反,离子簇优先在夹层溶液中形成(而不是在粘土表面)。簇通过单个离子的结合和聚集而增长,并在不同系统中以明显不同的效率吸附到粘土表面。发现蒙脱石在从溶液中捕获簇方面比高岭石更有效。然而,将离子簇锚定到粘土表面的效率在很大程度上取决于溶液的 Na+ 浓度,因为 Na+ 似乎强烈附着在表面上,从而阻止它与溶液中的簇连接。因此,蒙脱石(可能还有其他绿土粘土)可能在某些主要是淡水的系统中发挥重要作用,将离子簇定位在其表面,从而促进结晶碳酸钙镁矿物的成核和模板化生长。将离子簇固定到粘土表面的效率很大程度上取决于溶液的 Na+ 浓度,因为 Na+ 似乎强烈附着在表面上,从而阻止它与溶液中的簇接触。因此,蒙脱石(可能还有其他绿土粘土)可能在某些主要是淡水的系统中发挥重要作用,将离子簇定位在其表面,从而促进结晶碳酸钙镁矿物的成核和模板化生长。将离子簇固定到粘土表面的效率很大程度上取决于溶液的 Na+ 浓度,因为 Na+ 似乎强烈附着在表面上,从而阻止它与溶液中的簇接触。因此,蒙脱石(可能还有其他绿土粘土)可能在某些主要是淡水的系统中发挥重要作用,将离子簇定位在其表面,从而促进结晶碳酸钙镁矿物的成核和模板化生长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号