Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-02-08 , DOI: 10.1016/j.jphotochem.2020.112430 Sakshi Bhardwaj , Bonamali Pal
|
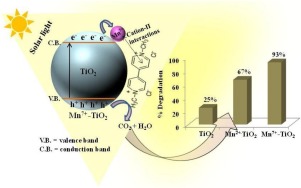
Transition metal/metal ions are reported to impart very efficient co-catalytic activity to TiO2 for various photocatalytic reactions depending on the nature and oxidation state of metal ions. However, when certain metals (Mn+) having different oxidation states like Mn2+/Mn7+ or Cr3+/Cr6+ act as co-catalysts, the photocatalytic properties of Mn+-TiO2 nanocomposites may vary significantly. Different surface morphologies, electron transfer phenomena and adsorption efficacy provided by these co-catalysts will govern the Mn+-TiO2 photoactivity. In this context, this paper demonstrates the preparation and characterization of Mn2+/Mn7+-TiO2 nanocomposites for investigating the above physicochemical properties for the degradation of toxic herbicide Methyl viologen under sunlight irradiation. The optical spectra of these catalysts showed bathochromic shifts (from 355 to 572 nm) due to the presence of Mn2+ and Mn7+ ions. The higher co-catalyst stability, smaller Dmax (maximum average diameter) and mixed morphological characteristics have been illustrated by SAXS and TEM analysis with size range ∼ 25–30 and 20−35 nm for Mn2+-TiO2 and Mn7+-TiO2 nanocomposites, respectively. The adsorption-desorption studies using NH3 gas revealed that Mn7+ ions generate enhanced Lewis acidity on the co-catalyst surface compared to Mn2+ impregnations. The best fit for Freundlich adsorption isotherm (Kf = 23.44 μg/mg) was possessed by Mn7+-TiO2 than Mn2+-TiO2 and TiO2 photocatalyst confirming its higher substrate affinity and photodegradation efficiency. The Mn2+ and Mn7+ impregnations displayed 2.6 and 3.7 times higher photoactivity than bare TiO2 which has been correlated to different oxidation states and surface structural properties imparted by Mnn+ ions.
中文翻译:

Mn 2+ / Mn 7+ -TiO 2纳米复合材料的太阳光驱动甲基紫精的光催化氧化降解
据报道,根据金属离子的性质和氧化态,对于各种光催化反应,过渡金属/金属离子可赋予TiO 2非常有效的助催化活性。但是,当某些具有不同氧化态的金属(M n +)像Mn 2+ / Mn 7+或Cr 3+ / Cr 6+充当助催化剂时,M n + -TiO 2纳米复合材料的光催化性能可能会发生显着变化。这些助催化剂提供的不同表面形态,电子转移现象和吸附效率将决定M n + -TiO 2光活性。在此背景下,本文证明了Mn 2+ / Mn 7+ -TiO 2纳米复合材料的制备和表征,以研究上述理化性质对日光照射下有毒除草剂甲基紫精的降解作用。由于存在Mn 2+和Mn 7+离子,这些催化剂的光谱显示出红移(从355 nm到572 nm)。通过SAXS和TEM分析,在Mn 2+ -TiO 2和Mn 7+的尺寸范围分别为25-30和20-35 nm的范围内,已证明了较高的助催化剂稳定性,较小的D max(最大平均直径)和混合的形态特征。-TiO 2纳米复合材料,分别。使用NH 3气体的吸附-解吸研究表明,与Mn 2+浸渍相比,Mn 7+离子在助催化剂表面产生增强的Lewis酸度。与Mn 2+ -TiO 2和TiO 2光催化剂相比 ,Mn 7+ -TiO 2具有最适合Freundlich吸附等温线的条件(K f = 23.44μg/ mg),证实了其较高的底物亲和力和光降解效率。Mn 2+和Mn 7+的浸渍显示的光活性是裸TiO 2的2.6和3.7倍这与Mn n +离子赋予的不同氧化态和表面结构特性有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号