Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-02-08 , DOI: 10.1016/j.jphotochem.2020.112426 B. Kirthika Rani , S. Abraham John
|
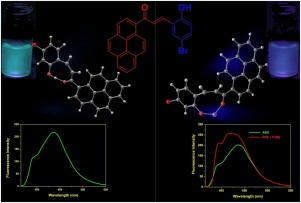
A novel fluorescent sensor for the selective and sensitive detection of Fe3+ based on fluorescent enhancement has been developed in the present study. The sensor 3-(4-bromo-2-hydroxyphenyl)-1-(pyren-1-yl)prop-2-en-1-one (ABS) was synthesized by Claisen-Schmidt reaction between acetyl pyrene and 5-bromosalicylaldehyde. It was characterized by FT-IR, HR-MS,1H and 13C NMR. The sensor exhibited weak fluorescence due to C = C isomerization and intramolecular charge transfer (ICT) process but upon binding with Fe3+ it exhibited increase in fluorescence due to blockage of ICT and C = C isomerization. While adding above 50 μM Fe3+, the probe exhibited decrease in fluorescence, as against the saturation curve. The decrease was ascribed to ligand to metal charge transfer (LMCT). This phenomenon of electron transfer from the fluorophore to the bound metal ion resulted in decrease of the fluorescence via non-radiative energy transfer. The binding constant was found to be 2 × 103 M−1. The binding mechanism was established based on theoretical calculations whereas the fluorescence enhancement and decrease were confirmed by quantum yield and life-time studies. The binding mode of ABS-Fe3+ complex was ascertained by using 1H NMR and DFT studies. When increasing Fe3+ concentration from 50 nM to 1 μM, the emission intensity was linearly increased against the concentration of Fe3+ with a correlation coefficient of 0.999 and LOD was found to be 11.2 nM (3σ/S). Finally, the probe was successfully employed to determine Fe3+ in real samples and cell imaging studies.
中文翻译:

半水溶液中具有a荧光基团的Fe(III)离子的选择性受体
本研究开发了一种新型的荧光传感器,用于基于荧光增强的Fe 3+的选择性和灵敏检测。传感器pyr3-(4-溴-2-羟基苯基)-1-(吡啶-1-基)丙-2-烯-1-酮(ABS)是通过乙酰C和5-溴水杨醛之间的克莱森-施密特反应合成的。通过FT-IR,HR-MS,1 H和13 C NMR对其进行表征。传感器由于C = C异构化和分子内电荷转移(ICT)过程而显示出弱荧光,但是与Fe 3+结合后,由于ICT和C = C异构化而使其荧光增强。加入超过50μM的Fe 3+,探针显示出荧光的减少,与饱和曲线相对。减少归因于配体到金属的电荷转移(LMCT)。电子从荧光团转移到结合的金属离子的现象导致荧光通过非辐射能量转移而减少。发现结合常数为2×10 3 M -1。根据理论计算建立了结合机理,而量子产率和寿命研究证实了荧光的增强和减弱。通过1 H NMR和DFT研究确定ABS-Fe 3+配合物的结合模式。当增加Fe 3+时从50 nM到1μM的浓度,发射强度相对于Fe 3+浓度线性增加,相关系数为0.999,LOD为11.2 nM(3σ/ S)。最后,该探针成功地用于测定实际样品和细胞成像研究中的Fe 3+。











































 京公网安备 11010802027423号
京公网安备 11010802027423号