Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microglial NMDA receptors drive pro-inflammatory responses via PARP-1/TRMP2 signaling.
Glia ( IF 5.4 ) Pub Date : 2020-02-08 , DOI: 10.1002/glia.23790 Prajwal Raghunatha 1, 2 , Amir Vosoughi 2 , Tiina M Kauppinen 1, 2, 3 , Michael F Jackson 1, 2
Glia ( IF 5.4 ) Pub Date : 2020-02-08 , DOI: 10.1002/glia.23790 Prajwal Raghunatha 1, 2 , Amir Vosoughi 2 , Tiina M Kauppinen 1, 2, 3 , Michael F Jackson 1, 2
Affiliation
|
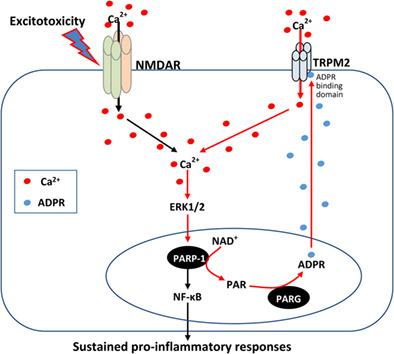
Chronic neuroinflammation driven by microglia is a characteristic feature associated with numerous neurodegenerative diseases. While acute inflammation can assist with recovery and repair, prolonged microglial pro-inflammatory responses are known to exacerbate neurodegenerative processes. Yet, detrimental outcomes of extended microglial activation are counterbalanced by beneficial outcomes including phagocytosis and release of trophic factors promoting neuronal viability. Our past work has shown that the nuclear enzyme poly(ADP-ribose) polymerase-1 (PARP-1) is a key signaling hub driving pro-inflammatory microglia responses, but the signaling pathway maintaining PARP-1 activation remains elusive. While best understood for its role in promoting DNA repair, our group has shown that PARP-1 activity can be stimulated via Ca2+ influx-dependent ERK1/2-mediated phosphorylation. However, to date, the route of Ca2+ entry responsible for stimulating PARP-1 has not been identified. A likely candidate is via Ca2+ -permeable transient receptor potential melastatin 2 (TRPM2) channels activated downstream of PARP-1 in a cascade that involves ADP-ribose (ADPR) production by poly(ADP-ribose) glycohydrolase (PARG). Here we demonstrate that NMDA receptor (NMDAR) stimulation in primary cultured microglia induces their proliferation, morphological activation and release of pro-inflammatory mediators. These responses were contingent on the recruitment of PARP-1, PARG and Ca2+ permeable TRPM2 channels. Furthermore, we show that Ca2+ influx is necessary to activate PARP-1/TRPM2 signaling, in an ERK1/2-dependent, but DNA damage independent, manner. Our findings, showing that PARP-1/TRPM2 mediate the pro-inflammatory effects of NMDAR stimulation, provides a unifying mechanism linking elevated glutamate levels to chronic neuroinflammation.
中文翻译:

小胶质细胞 NMDA 受体通过 PARP-1/TRMP2 信号驱动促炎反应。
由小胶质细胞驱动的慢性神经炎症是与许多神经退行性疾病相关的特征。虽然急性炎症可以帮助恢复和修复,但已知长时间的小胶质细胞促炎反应会加剧神经退行性过程。然而,延长小胶质细胞激活的不利结果被有益结果抵消,包括吞噬作用和促进神经元活力的营养因子的释放。我们过去的工作表明,核酶聚(ADP-核糖)聚合酶-1(PARP-1)是驱动促炎小胶质细胞反应的关键信号枢纽,但维持 PARP-1 激活的信号通路仍然难以捉摸。虽然最好理解它在促进 DNA 修复方面的作用,我们的小组已经表明 PARP-1 活性可以通过 Ca2+ 流入依赖性 ERK1/2 介导的磷酸化来刺激。然而,迄今为止,负责刺激 PARP-1 的 Ca2+ 进入途径尚未确定。一个可能的候选者是通过 PARP-1 下游激活的 Ca2+ 可渗透瞬时受体电位 melastatin 2 (TRPM2) 通道,级联反应涉及聚 (ADP-核糖) 糖水解酶 (PARG) 产生的 ADP-核糖 (ADPR)。在这里,我们证明原代培养的小胶质细胞中的 NMDA 受体 (NMDAR) 刺激会诱导它们的增殖、形态激活和促炎介质的释放。这些反应取决于 PARP-1、PARG 和 Ca2+ 可渗透 TRPM2 通道的募集。此外,我们表明 Ca2+ 流入是激活 PARP-1/TRPM2 信号传导所必需的,在依赖于 ERK1/2 的情况下,但DNA损伤独立,方式。我们的研究结果表明,PARP-1/TRPM2 介导了 NMDAR 刺激的促炎作用,提供了将谷氨酸水平升高与慢性神经炎症联系起来的统一机制。
更新日期:2020-02-08
中文翻译:

小胶质细胞 NMDA 受体通过 PARP-1/TRMP2 信号驱动促炎反应。
由小胶质细胞驱动的慢性神经炎症是与许多神经退行性疾病相关的特征。虽然急性炎症可以帮助恢复和修复,但已知长时间的小胶质细胞促炎反应会加剧神经退行性过程。然而,延长小胶质细胞激活的不利结果被有益结果抵消,包括吞噬作用和促进神经元活力的营养因子的释放。我们过去的工作表明,核酶聚(ADP-核糖)聚合酶-1(PARP-1)是驱动促炎小胶质细胞反应的关键信号枢纽,但维持 PARP-1 激活的信号通路仍然难以捉摸。虽然最好理解它在促进 DNA 修复方面的作用,我们的小组已经表明 PARP-1 活性可以通过 Ca2+ 流入依赖性 ERK1/2 介导的磷酸化来刺激。然而,迄今为止,负责刺激 PARP-1 的 Ca2+ 进入途径尚未确定。一个可能的候选者是通过 PARP-1 下游激活的 Ca2+ 可渗透瞬时受体电位 melastatin 2 (TRPM2) 通道,级联反应涉及聚 (ADP-核糖) 糖水解酶 (PARG) 产生的 ADP-核糖 (ADPR)。在这里,我们证明原代培养的小胶质细胞中的 NMDA 受体 (NMDAR) 刺激会诱导它们的增殖、形态激活和促炎介质的释放。这些反应取决于 PARP-1、PARG 和 Ca2+ 可渗透 TRPM2 通道的募集。此外,我们表明 Ca2+ 流入是激活 PARP-1/TRPM2 信号传导所必需的,在依赖于 ERK1/2 的情况下,但DNA损伤独立,方式。我们的研究结果表明,PARP-1/TRPM2 介导了 NMDAR 刺激的促炎作用,提供了将谷氨酸水平升高与慢性神经炎症联系起来的统一机制。









































 京公网安备 11010802027423号
京公网安备 11010802027423号