当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trialkylsilyl-Substituted Silole and Germole Dianions as Precursors for Unusual Silicon and Germanium Compounds.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-02-07 , DOI: 10.1021/acs.accounts.9b00636 Zhaowen Dong 1 , Lena Albers 1 , Thomas Müller 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-02-07 , DOI: 10.1021/acs.accounts.9b00636 Zhaowen Dong 1 , Lena Albers 1 , Thomas Müller 1
Affiliation
|
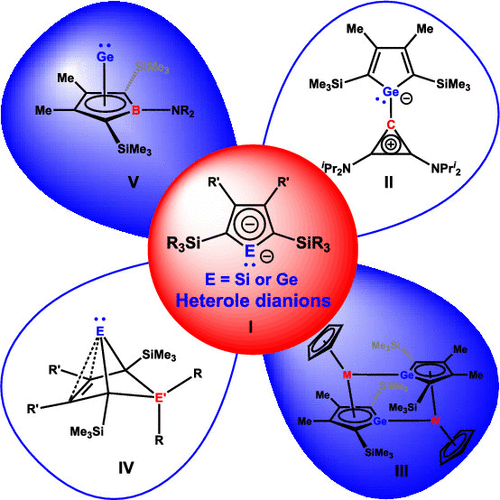
Group 14 element heteroles are the heavier analogues of cyclopentadienes in which a heavier group 14 element atom replaces the sp3 carbon atom. In particular siloles and, to a somewhat smaller degree, germoles attracted considerable attention since the early 1990s due to their favorable photophysical properties which allowed the construction of OLEDs using group 14 element heteroles as emissive or electron-transport layers. Anions and in particular dianions derived from group 14 element heteroles have been of substantial interest due to the possible occurrence of Hückel aromaticity involving the heavier main group atom. Aromaticity is not the only notable electronic feature of silole and germole dianions; the spatial and energetic alignment of their frontier orbitals is equally remarkable. With a high lying lone pair at the heteroatom, which is orthogonal to a delocalized π-system, their frontier orbital sequence closely resembles that of N-heterocyclic carbene analogues. Despite these intriguing parallels between carbene analogues and silole and germole dianions, disappointingly little is known about their reactivity. The installation of trialkylsilyl substituents in the 2,5-positions of the heterocyclopentadiene ring as in K2[I] has a remarkable effect on the stability of silole and germole dianions and allows us to study their reactivity and to evaluate their synthetic potential in detail. Simple double salt metathesis reactions with different dihalides provided heterofulvenes. These were detected either as intermediates or, in the case of carbon dihalides, isolated in the form of their ylidic isomers II. In other cases, the heterofulvenes were the starting point for complex reaction sequences leading to novel binuclear complexes of titanium and zirconium III or for simple isomerization reactions that lead to bicyclohexene-type tetrylenes (BCH-tetrylenes) IV, a novel class of heavier carbenes. These bicyclic carbene analogues are significantly stabilized by homoconjugation between the electron deficient tetrel atom and the remote C═C double bond. Compound IV with E'R2═SiR2 and E = Si is a valence isomer of disilabenzene and is a stable derivative of the global minimum of the Si2C4H6 potential energy surface. With group 13 dihalides, as for example with boron dichlorides, topological closely related compounds V were isolated. These Ge(II) complexes of borole dianions are isolobal to half-sandwich complexes of main group elements such as aluminum(I) cyclopentadienide or can be viewed as nido-type clusters. These analogies already open a broad field for future investigations of their reactivity. Trialkylsilyl-substituted heterole dianions I provide a facile synthetic approach to several novel intriguing compound classes with the tetrel element in unusual coordination states. The reactivity and the synthetic potential of these new compounds is however widely unexplored and calls for future systematic studies. Gratifyingly, the periodic table of the elements stills holds a lot of potential for future research on the reactivity of silole and germole dianions.
中文翻译:

三烷基甲硅烷基取代的Silole和Germole阴离子作为异常硅和锗化合物的前体。
14族元素杂环是环戊二烯的较重类似物,其中较重的14族元素原子取代了sp3碳原子。自1990年代初以来,尤其是硅微石,并且在某种程度上较小,胚芽因其良好的光物理性质而受到了广泛的关注,这使得它们能够使用14族元素杂环作为发射层或电子传输层来构造OLED。由于可能会出现涉及较重的主原子的Hückel芳族化合物,因此引起人们广泛关注的是源自第14组元素杂原子的阴离子,尤其是二价阴离子。芳香性不是唯一的硅化物和胚芽二价电子特征。它们的前沿轨道在空间和能量上的排列同样引人注目。在杂原子上有一个高躺的孤对,它与离域π系统正交,其前沿轨道序列与N杂环卡宾类似物的轨道序列极为相似。尽管在卡宾类似物与甲硅烷基和生殖烯二价阴离子之间存在这些有趣的相似之处,但令人失望的是,它们的反应性知之甚少。如K2 [I]所示,在杂环戊二烯环的2,5-位上安装三烷基甲硅烷基取代基对甲硅烷基二烯和锗烯二价阴离子的稳定性有显着影响,这使我们能够研究它们的反应性并详细评估其合成潜力。与不同的二卤化物进行简单的复盐复分解反应提供了异富烯。它们被检测为中间体,或者在二卤化碳的情况下,以其旋状异构体II的形式被分离出来。在其他情况下,杂富烯是引发钛和锆III的新型双核络合物的复杂反应序列的起点,或者是导致新型双环己烯型四环戊二烯型四甲苯(BCH-四甲苯)IV的简单异构化反应的起点。这些双环卡宾类似物通过缺电子的蝶形原子与远端C═C双键之间的均共轭作用而显着稳定。具有E'R2═SiR2和E = Si的化合物IV是二硅苯的化合价异构体,是Si2C4H6势能面整体最小值的稳定衍生物。对于第13族二卤化物,例如对于二氯化硼,分离出拓扑密切相关的化合物V。这些硼化双阴离子的Ge(II)络合物是主要族元素(例如环戊二烯酸铝(I))的等三明治至半夹心络合物,或者可以看作是Nido型团簇。这些类比已经为未来对其反应性的研究开辟了广阔的领域。三烷基甲硅烷基取代的杂芳族二价化合物I提供了一种简便的合成方法,可对几种新颖有趣的化合物类别(其中的蝶形元素处于不寻常的配位态)进行合成。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进行进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。这些类比已经为未来对其反应性的研究开辟了广阔的领域。三烷基甲硅烷基取代的杂环二价阴离子提供了一种简便的合成方法,可对几种新颖的有趣化合物类别(其中的铁et元素处于异常配位态)进行合成。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进行进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。这些类比已经为未来对其反应性的研究开辟了广阔的领域。三烷基甲硅烷基取代的杂芳族二价化合物I提供了一种简便的合成方法,可对几种新颖有趣的化合物类别(其中的蝶形元素处于不寻常的配位态)进行合成。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进行进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进行进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。
更新日期:2020-02-10
中文翻译:

三烷基甲硅烷基取代的Silole和Germole阴离子作为异常硅和锗化合物的前体。
14族元素杂环是环戊二烯的较重类似物,其中较重的14族元素原子取代了sp3碳原子。自1990年代初以来,尤其是硅微石,并且在某种程度上较小,胚芽因其良好的光物理性质而受到了广泛的关注,这使得它们能够使用14族元素杂环作为发射层或电子传输层来构造OLED。由于可能会出现涉及较重的主原子的Hückel芳族化合物,因此引起人们广泛关注的是源自第14组元素杂原子的阴离子,尤其是二价阴离子。芳香性不是唯一的硅化物和胚芽二价电子特征。它们的前沿轨道在空间和能量上的排列同样引人注目。在杂原子上有一个高躺的孤对,它与离域π系统正交,其前沿轨道序列与N杂环卡宾类似物的轨道序列极为相似。尽管在卡宾类似物与甲硅烷基和生殖烯二价阴离子之间存在这些有趣的相似之处,但令人失望的是,它们的反应性知之甚少。如K2 [I]所示,在杂环戊二烯环的2,5-位上安装三烷基甲硅烷基取代基对甲硅烷基二烯和锗烯二价阴离子的稳定性有显着影响,这使我们能够研究它们的反应性并详细评估其合成潜力。与不同的二卤化物进行简单的复盐复分解反应提供了异富烯。它们被检测为中间体,或者在二卤化碳的情况下,以其旋状异构体II的形式被分离出来。在其他情况下,杂富烯是引发钛和锆III的新型双核络合物的复杂反应序列的起点,或者是导致新型双环己烯型四环戊二烯型四甲苯(BCH-四甲苯)IV的简单异构化反应的起点。这些双环卡宾类似物通过缺电子的蝶形原子与远端C═C双键之间的均共轭作用而显着稳定。具有E'R2═SiR2和E = Si的化合物IV是二硅苯的化合价异构体,是Si2C4H6势能面整体最小值的稳定衍生物。对于第13族二卤化物,例如对于二氯化硼,分离出拓扑密切相关的化合物V。这些硼化双阴离子的Ge(II)络合物是主要族元素(例如环戊二烯酸铝(I))的等三明治至半夹心络合物,或者可以看作是Nido型团簇。这些类比已经为未来对其反应性的研究开辟了广阔的领域。三烷基甲硅烷基取代的杂芳族二价化合物I提供了一种简便的合成方法,可对几种新颖有趣的化合物类别(其中的蝶形元素处于不寻常的配位态)进行合成。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进行进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。这些类比已经为未来对其反应性的研究开辟了广阔的领域。三烷基甲硅烷基取代的杂环二价阴离子提供了一种简便的合成方法,可对几种新颖的有趣化合物类别(其中的铁et元素处于异常配位态)进行合成。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进行进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。这些类比已经为未来对其反应性的研究开辟了广阔的领域。三烷基甲硅烷基取代的杂芳族二价化合物I提供了一种简便的合成方法,可对几种新颖有趣的化合物类别(其中的蝶形元素处于不寻常的配位态)进行合成。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进行进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。然而,这些新化合物的反应性和合成潜能尚未得到广泛探索,需要进行进一步的系统研究。令人高兴的是,元素周期表仍然具有很大的潜力,可用于今后对硅酮和胚芽二价阴离子反应性的研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号