Gastroenterology ( IF 25.7 ) Pub Date : 2020-02-08 , DOI: 10.1053/j.gastro.2020.01.047 William J Sandborn 1 , Brian G Feagan 2 , Edward V Loftus 3 , Laurent Peyrin-Biroulet 4 , Gert Van Assche 5 , Geert D'Haens 6 , Stefan Schreiber 7 , Jean-Frederic Colombel 8 , James D Lewis 9 , Subrata Ghosh 10 , Alessandro Armuzzi 11 , Ellen Scherl 12 , Hans Herfarth 13 , Lauren Vitale 14 , Mohamed-Eslam F Mohamed 14 , Ahmed A Othman 14 , Qian Zhou 14 , Bidan Huang 14 , Roopal B Thakkar 14 , Aileen L Pangan 14 , Ana P Lacerda 14 , Julian Panes 15
|
Background & Aims
We evaluated the efficacy and safety of upadacitinib, an oral selective Janus kinase 1 inhibitor, in a randomized trial of patients with Crohn’s disease (CD).
Methods
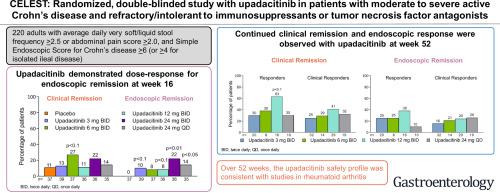
We performed a double-blind, phase 2 trial in adults with moderate to severe CD and inadequate response or intolerance to immunosuppressants or tumor necrosis factor antagonists. Patients were randomly assigned (1:1:1:1:1:1) to groups given placebo; or 3 mg, 6 mg, 12 mg, or 24 mg upadacitinib twice daily; or 24 mg upadacitinib once daily and were evaluated by ileocolonoscopy at weeks 12 or 16 of the induction period. Patients who completed week 16 were re-randomized to a 36-week period of maintenance therapy with upadacitinib. The primary endpoints were clinical remission at week 16 and endoscopic remission at week 12 or 16 using the multiple comparison procedure and modeling and the Cochran-Mantel-Haenszel test, with a 2-sided level of 10%.
Results
Among the 220 patients in the study, clinical remission was achieved by 13% of patients receiving 3 mg upadacitinib, 27% of patients receiving 6 mg upadacitinib (P < .1 vs placebo), 11% of patients receiving 12 mg upadacitinib, and 22% of patients receiving 24 mg upadacitinib twice daily, and by 14% of patients receiving 24 mg upadacitinib once daily, vs 11% of patients receiving placebo. Endoscopic remission was achieved by 10% (P < .1 vs placebo), 8%, 8% (P < .1 vs placebo), 22% (P < .01 vs placebo), and 14% (P < .05 vs placebo) of patients receiving upadacitinib, respectively, vs none of the patients receiving placebo. Endoscopic but not clinical remission increased with dose during the induction period. Efficacy was maintained for most endpoints through week 52. During the induction period, patients in the upadacitinib groups had higher incidences of infections and serious infections vs placebo. Patients in the twice-daily 12 mg and 24 mg upadacitinib groups had significant increases in total, high-density lipoprotein, and low-density lipoprotein cholesterol levels compared with patients in the placebo group.
Conclusions
In a phase 2 trial of patients with CD, upadacitinib induced endoscopic remission in a significant proportion of patients compared with placebo. Upadacitinib’s benefit/risk profile supports further development for treatment of CD. (Clinicaltrials.gov, Number: NCT02365649)
中文翻译:

Upadacitinib 在克罗恩病患者随机试验中的疗效和安全性。
背景与目标
我们在一项针对克罗恩病 (CD) 患者的随机试验中评估了 upadacitinib(一种口服选择性 Janus 激酶 1 抑制剂)的有效性和安全性。
方法
我们在患有中度至重度 CD 且对免疫抑制剂或肿瘤坏死因子拮抗剂反应不足或不耐受的成人中进行了一项双盲 2 期试验。患者被随机分配(1:1:1:1:1:1)到安慰剂组;或 3 毫克、6 毫克、12 毫克或 24 毫克 upadacitinib,每日两次;或 24 mg upadacitinib 每日一次,并在诱导期的第 12 周或第 16 周通过回结肠镜检查进行评估。完成第 16 周的患者被重新随机分配接受 upadacitinib 为期 36 周的维持治疗。主要终点是第 16 周的临床缓解和第 12 或 16 周的内窥镜缓解,使用多重比较程序和建模以及 Cochran-Mantel-Haenszel 检验,2 侧水平为 10%。
结果
在该研究的 220 名患者中,接受 3 mg upadacitinib 的患者中有 13% 达到临床缓解,接受 6 mg upadacitinib 的患者为 27%(P < .1 vs 安慰剂),接受 12 mg upadacitinib 的患者为 11%,以及 22每天两次接受 24 mg upadacitinib 的患者的百分比,以及每天一次接受 24 mg upadacitinib 的患者的 14%,而接受安慰剂的患者的比例为 11%。内镜下的缓解率分别为 10%(P < .1 与安慰剂)、8%、8%(P < .1 与安慰剂)、22%(P < .01 与安慰剂)和 14%(P <.05 vs 安慰剂)分别是接受 upadacitinib 的患者,而没有接受安慰剂的患者。在诱导期内,内镜但非临床缓解随剂量增加而增加。大多数终点的疗效一直持续到第 52 周。在诱导期内,upadacitinib 组的患者与安慰剂相比,感染和严重感染的发生率更高。与安慰剂组患者相比,每天两次 12 毫克和 24 毫克 upadacitinib 组的患者的总胆固醇、高密度脂蛋白和低密度脂蛋白胆固醇水平显着增加。
结论
在 CD 患者的 2 期试验中,与安慰剂相比,upadacitinib 诱导了相当一部分患者的内镜缓解。Upadacitinib 的获益/风险状况支持 CD 治疗的进一步发展。(Clinicaltrials.gov,编号:NCT02365649)











































 京公网安备 11010802027423号
京公网安备 11010802027423号