Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The effect of assessing genetic risk of prostate cancer on the use of PSA tests in primary care: A cluster randomized controlled trial.
PLOS Medicine ( IF 10.5 ) Pub Date : 2020-02-07 , DOI: 10.1371/journal.pmed.1003033 Jacob Fredsøe 1, 2 , Jan Koetsenruyter 3 , Peter Vedsted 1, 3 , Pia Kirkegaard 4 , Michael Væth 5 , Adrian Edwards 6 , Torben F Ørntoft 1, 2 , Karina D Sørensen 1, 2 , Flemming Bro 3
PLOS Medicine ( IF 10.5 ) Pub Date : 2020-02-07 , DOI: 10.1371/journal.pmed.1003033 Jacob Fredsøe 1, 2 , Jan Koetsenruyter 3 , Peter Vedsted 1, 3 , Pia Kirkegaard 4 , Michael Væth 5 , Adrian Edwards 6 , Torben F Ørntoft 1, 2 , Karina D Sørensen 1, 2 , Flemming Bro 3
Affiliation
|
BACKGROUND
Assessing genetic lifetime risk for prostate cancer has been proposed as a means of risk stratification to identify those for whom prostate-specific antigen (PSA) testing is likely to be most valuable. This project aimed to test the effect of introducing a genetic test for lifetime risk of prostate cancer in general practice on future PSA testing.
METHODS AND FINDINGS
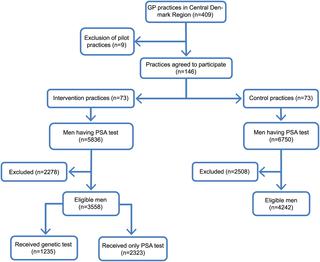
We performed a cluster randomized controlled trial with randomization at the level of general practices (73 in each of two arms) in the Central Region (Region Midtjylland) of Denmark. In intervention practices, men were offered a genetic test (based on genotyping of 33 risk-associated single nucleotide polymorphisms) in addition to the standard PSA test that informed them about lifetime genetic risk of prostate cancer and distinguished between "normal" and "high" risk. The primary outcome was the proportion of men having a repeated PSA test within 2 years. A multilevel logistic regression model was used to test the association. After applying the exclusion criteria, 3,558 men were recruited in intervention practices, with 1,235 (34.7%) receiving the genetic test, and 4,242 men were recruited in control practices. Men with high genetic risk had a higher propensity for repeated PSA testing within 2 years than men with normal genetic risk (odds ratio [OR] = 8.94, p < 0.01). The study was conducted in routine practice and had some selection bias, which is evidenced by the relatively large proportion of younger and higher income participants taking the genetic test.
CONCLUSIONS
Providing general practitioners (GPs) with access to a genetic test to assess lifetime risk of prostate cancer did not reduce the overall number of future PSA tests. However, among men who had a genetic test, knowledge of genetic risk significantly influenced future PSA testing.
TRIAL REGISTRATION
This study is registered with ClinicalTrials.gov, number NCT01739062.
中文翻译:

评估前列腺癌遗传风险对初级保健中使用 PSA 测试的影响:一项整群随机对照试验。
背景 评估前列腺癌的遗传终生风险已被提议作为一种风险分层手段,以识别那些前列腺特异性抗原(PSA)测试可能最有价值的人。该项目旨在测试在一般实践中引入前列腺癌终生风险基因检测对未来 PSA 检测的影响。方法和结果 我们在丹麦中部地区(中日德兰地区)进行了一项整群随机对照试验,在全科实践水平上进行随机化(两组各 73 例)。在干预实践中,除了标准 PSA 测试之外,还为男性提供了基因测试(基于 33 种风险相关单核苷酸多态性的基因分型),该测试告知他们前列腺癌的终生遗传风险并区分“正常”和“高”风险。主要结果是两年内重复进行 PSA 测试的男性比例。使用多级逻辑回归模型来测试关联性。应用排除标准后,干预实践中招募了 3,558 名男性,其中 1,235 人(34.7%)接受了基因检测,对照实践中招募了 4,242 名男性。具有高遗传风险的男性比具有正常遗传风险的男性在 2 年内重复进行 PSA 检测的倾向更高(比值比 [OR] = 8.94,p < 0.01)。该研究是在常规实践中进行的,存在一定的选择偏差,接受基因测试的年轻和高收入参与者比例相对较大就证明了这一点。结论 为全科医生 (GP) 提供基因检测来评估终生患前列腺癌的风险并不会减少未来 PSA 检测的总数。 然而,在接受过基因检测的男性中,遗传风险知识显着影响了未来的 PSA 检测。试验注册 本研究已在 ClinicalTrials.gov 注册,注册号为 NCT01739062。
更新日期:2020-02-07
中文翻译:

评估前列腺癌遗传风险对初级保健中使用 PSA 测试的影响:一项整群随机对照试验。
背景 评估前列腺癌的遗传终生风险已被提议作为一种风险分层手段,以识别那些前列腺特异性抗原(PSA)测试可能最有价值的人。该项目旨在测试在一般实践中引入前列腺癌终生风险基因检测对未来 PSA 检测的影响。方法和结果 我们在丹麦中部地区(中日德兰地区)进行了一项整群随机对照试验,在全科实践水平上进行随机化(两组各 73 例)。在干预实践中,除了标准 PSA 测试之外,还为男性提供了基因测试(基于 33 种风险相关单核苷酸多态性的基因分型),该测试告知他们前列腺癌的终生遗传风险并区分“正常”和“高”风险。主要结果是两年内重复进行 PSA 测试的男性比例。使用多级逻辑回归模型来测试关联性。应用排除标准后,干预实践中招募了 3,558 名男性,其中 1,235 人(34.7%)接受了基因检测,对照实践中招募了 4,242 名男性。具有高遗传风险的男性比具有正常遗传风险的男性在 2 年内重复进行 PSA 检测的倾向更高(比值比 [OR] = 8.94,p < 0.01)。该研究是在常规实践中进行的,存在一定的选择偏差,接受基因测试的年轻和高收入参与者比例相对较大就证明了这一点。结论 为全科医生 (GP) 提供基因检测来评估终生患前列腺癌的风险并不会减少未来 PSA 检测的总数。 然而,在接受过基因检测的男性中,遗传风险知识显着影响了未来的 PSA 检测。试验注册 本研究已在 ClinicalTrials.gov 注册,注册号为 NCT01739062。









































 京公网安备 11010802027423号
京公网安备 11010802027423号