The Journal of Supercritical Fluids ( IF 3.4 ) Pub Date : 2020-02-07 , DOI: 10.1016/j.supflu.2020.104783 Zongliang Qiao , Yue Cao , Yuming Yin , Lingling Zhao , Fengqi Si
|
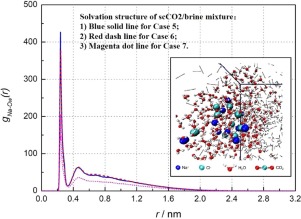
This paper reports an investigation for solvation structure of supercritical CO2 (scCO2) and brine mixture for CO2 plume geothermal (CPG) applications. Molecular dynamics simulations are performed to calculate radial distribution function, coordination number and hydrogen-bond number for mixtures of different compositions. This study can help reveal the solvation structures of scCO2/brine mixture under different temperatures (373.15 K–473.15 K) and ion concentrations (1 wt% to 10 wt%). Results show that H2O molecules bind more tightly to Na+ ion than Cl− ion while scCO2 molecules seem have a uniform distribution in the scCO2/brine mixture. Both the interaction of Na+-H2O pairs and that of Cl--H2O pairs become stronger under higher temperature conditions. Besides, the increase of ion concentration not only reduces the number of H2O molecules in the solvation shell of Na+ ion, but also weakens the interaction of hydrogen bonds. Moreover, it seems that the scCO2/brine mixture is heterogeneous in the CPG application.
中文翻译:

用于CO 2羽地热应用的超临界CO 2和盐水混合物的溶剂化结构:分子动力学研究
本文报道了针对CO 2羽状地热(CPG)应用的超临界CO 2(scCO 2)和盐水混合物的溶剂化结构的研究。进行分子动力学模拟以计算不同组成的混合物的径向分布函数,配位数和氢键数。这项研究可以帮助揭示scCO 2 /盐水混合物在不同温度(373.15 K–473.15 K)和离子浓度(1 wt%至10 wt%)下的溶剂化结构。结果表明使得h 2水分子结合更紧密地与Na +离子比氯-离子而SCCO 2分子似乎具有在SCCO均匀分布2 /盐水混合物。在高温条件下,Na + -H 2 O对和Cl-H 2 O对的相互作用都更强。此外,离子浓度的增加不仅减少了Na +离子的溶剂化壳中H 2 O分子的数量,而且削弱了氢键的相互作用。而且,在CPG应用中似乎scCO 2 /盐水混合物是异质的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号