Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-02-07 , DOI: 10.1016/j.jphotochem.2020.112415 Rodrigo Domínguez , Angel Anzani , Matias Berasategui , Lucia Lanfri , Maxi A. Burgos Paci , Walter Peláez , Gustavo Argüello , Ana Iriarte
|
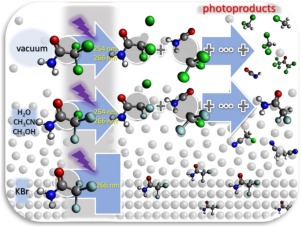
In this work we present the study of the direct photolysis processes for 2,2,2-trichloroacetamide (TCA), 2-chloro-2, 2-difluoroacetamide (CDFA) and 2,2,2-trifluoroacetamide (TFA), in solid state and solution. CH3OH, CH3CN and H2O were used as solvents, and the irradiation (254 nm) was carried out in oxygen enriched as well as in oxygen free (N2 bubbled) solutions. The 266 nm light was reserved for the solid samples. In solution, the process was followed by UV–vis spectroscopy and the products analyzed by gas chromatography coupled to mass spectrometry (GC MS); while the gas phase products from the solid photodegradation processes, were followed by FTIR spectroscopy. The main electronic transitions were assigned on the bases of chemical calculations, and they do corroborate the proposed mechanisms. For both TCA and CDFA, the breakdown of the C-Cl bond is observed as the major process, with an activation energy of around 42 kcal/mol (in solution) and 27 kcal/mol (in gas phase), and a quantum yield of 0.01. TFA does not degrade under these experimental conditions.
MS); while the gas phase products from the solid photodegradation processes, were followed by FTIR spectroscopy. The main electronic transitions were assigned on the bases of chemical calculations, and they do corroborate the proposed mechanisms. For both TCA and CDFA, the breakdown of the C-Cl bond is observed as the major process, with an activation energy of around 42 kcal/mol (in solution) and 27 kcal/mol (in gas phase), and a quantum yield of 0.01. TFA does not degrade under these experimental conditions.
中文翻译:

阐明了固态(266 nm)和溶液(254 nm)的CCl 3 C(O)NH 2,CClF 2 C(O)NH 2和CF 3 C(O)NH 2的光降解机理。实验和理论研究
在这项工作中,我们介绍了固体中2,2,2-三氯乙酰胺(TCA),2-氯-2、2-二氟乙酰胺(CDFA)和2,2,2-三氟乙酰胺(TFA)直接光解过程的研究状态和解决方案。使用CH 3 OH,CH 3 CN和H 2 O作为溶剂,并且在富氧和无氧(鼓泡N 2的)溶液中进行辐照(254 nm)。266 nm的光保留给固体样品。在溶液中,随后进行紫外可见光谱分析,并通过气相色谱-质谱联用(GC)分析产品 多发性硬化症); 固相光降解过程中产生的气相产物,然后进行FTIR光谱分析。主要的电子跃迁是在化学计算的基础上分配的,它们确实证实了所提出的机理。对于TCA和CDFA,C-Cl键的分解都是主要过程,活化能约为42 kcal / mol(在溶液中)和27 kcal / mol(在气相中),且量子产率高为0.01。在这些实验条件下,TFA不会降解。
多发性硬化症); 固相光降解过程中产生的气相产物,然后进行FTIR光谱分析。主要的电子跃迁是在化学计算的基础上分配的,它们确实证实了所提出的机理。对于TCA和CDFA,C-Cl键的分解都是主要过程,活化能约为42 kcal / mol(在溶液中)和27 kcal / mol(在气相中),且量子产率高为0.01。在这些实验条件下,TFA不会降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号