当前位置:
X-MOL 学术
›
Toxicology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Fusarium mycotoxin, 2-Amino-14,16-dimethyloctadecan-3-ol (AOD) induces vacuolization in HepG2 cells.
Toxicology ( IF 4.8 ) Pub Date : 2020-02-07 , DOI: 10.1016/j.tox.2020.152405 A Solhaug 1 , M L Torgersen 2 , J A Holme 3 , J Wiik-Nilsen 1 , B Thiede 4 , G S Eriksen 1
Toxicology ( IF 4.8 ) Pub Date : 2020-02-07 , DOI: 10.1016/j.tox.2020.152405 A Solhaug 1 , M L Torgersen 2 , J A Holme 3 , J Wiik-Nilsen 1 , B Thiede 4 , G S Eriksen 1
Affiliation
|
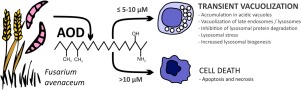
The mycotoxin 2-Amino-14,16-dimethyloctadecan-3-ol (AOD) has been isolated from cultures of the fungus Fusarium avenaceum, one of the most prevalent Fusarium species. AOD is an analogue of sphinganine and 1-deoxysphinganine, important intermediates in the de novo biosynthesis of cellular sphingolipids. Here we studied cellular effects of AOD using the human liver cell line HepG2 as a model system. AOD (10 μM) induced a transient accumulation of vacuoles in the cells. The effect was observed at non-cytotoxic concentrations and was not linked to cell death processes. Proteomic analyses indicated that protein degradation and/or vesicular transport may be a target for AOD. Further studies revealed that AOD had only minor effects on the initiation rate of macropinocytosis and autophagy. However, the AOD-induced vacuoles were lysosomal-associated membrane protein-1 (LAMP-1) positive, suggesting that they most likely originate from lysosomes or late endosomes. Accordingly, both endosomal and autophagic protein degradation were inhibited. Further studies revealed that treatment with concanamycin A or chloroquine completely blocked the AOD-induced vacuolization, suggesting that the vacuolization is dependent of acidic lysosomes. Overall, the results strongly suggest that the increased vacuolization is due to an accumulation of AOD in lysosomes or late endosomes thereby disturbing the later stages of the endolysosomal process.
中文翻译:

镰孢霉菌毒素2-氨基-14,16-二甲基十八烷-3-醇(AOD)诱导HepG2细胞中的空泡化。
从真菌镰刀菌(Fusarium avenaceum)(一种最普遍的镰刀菌属)的培养物中分离了霉菌毒素2-氨基-14,16-二甲基十八烷-3-醇(AOD)。AOD是Sphinganine和1-deoxysphinganine的类似物,它们是细胞鞘脂从头开始生物合成的重要中间体。在这里,我们使用人类肝细胞系HepG2作为模型系统研究了AOD的细胞作用。AOD(10μM)诱导细胞内液泡的短暂积累。在非细胞毒性浓度下观察到该作用,且与细胞死亡过程无关。蛋白质组学分析表明蛋白质降解和/或囊泡运输可能是AOD的目标。进一步的研究表明,AOD对巨噬细胞增多和自噬的起始速率影响很小。然而,AOD诱导的液泡是溶酶体相关膜蛋白1(LAMP-1)阳性,表明它们很可能起源于溶酶体或晚期内体。因此,内体和自噬蛋白降解均被抑制。进一步的研究表明,用刀豆球蛋白A或氯喹处理完全阻断了AOD诱导的空泡作用,表明该空泡作用取决于酸性溶酶体。总的来说,该结果强烈表明空泡化的增加是由于溶酶体或晚期内体中AOD的积累,从而干扰了溶酶体过程的后期。进一步的研究表明,用刀豆球蛋白A或氯喹处理完全阻断了AOD诱导的空泡作用,表明该空泡作用取决于酸性溶酶体。总的来说,该结果强烈表明空泡化的增加是由于溶酶体或晚期内体中AOD的积累,从而干扰了溶酶体过程的后期。进一步的研究表明,用刀豆球蛋白A或氯喹处理完全阻断了AOD诱导的空泡作用,表明该空泡作用取决于酸性溶酶体。总的来说,该结果强烈表明空泡化的增加是由于溶酶体或晚期内体中AOD的积累,从而干扰了溶酶体过程的后期。
更新日期:2020-02-07
中文翻译:

镰孢霉菌毒素2-氨基-14,16-二甲基十八烷-3-醇(AOD)诱导HepG2细胞中的空泡化。
从真菌镰刀菌(Fusarium avenaceum)(一种最普遍的镰刀菌属)的培养物中分离了霉菌毒素2-氨基-14,16-二甲基十八烷-3-醇(AOD)。AOD是Sphinganine和1-deoxysphinganine的类似物,它们是细胞鞘脂从头开始生物合成的重要中间体。在这里,我们使用人类肝细胞系HepG2作为模型系统研究了AOD的细胞作用。AOD(10μM)诱导细胞内液泡的短暂积累。在非细胞毒性浓度下观察到该作用,且与细胞死亡过程无关。蛋白质组学分析表明蛋白质降解和/或囊泡运输可能是AOD的目标。进一步的研究表明,AOD对巨噬细胞增多和自噬的起始速率影响很小。然而,AOD诱导的液泡是溶酶体相关膜蛋白1(LAMP-1)阳性,表明它们很可能起源于溶酶体或晚期内体。因此,内体和自噬蛋白降解均被抑制。进一步的研究表明,用刀豆球蛋白A或氯喹处理完全阻断了AOD诱导的空泡作用,表明该空泡作用取决于酸性溶酶体。总的来说,该结果强烈表明空泡化的增加是由于溶酶体或晚期内体中AOD的积累,从而干扰了溶酶体过程的后期。进一步的研究表明,用刀豆球蛋白A或氯喹处理完全阻断了AOD诱导的空泡作用,表明该空泡作用取决于酸性溶酶体。总的来说,该结果强烈表明空泡化的增加是由于溶酶体或晚期内体中AOD的积累,从而干扰了溶酶体过程的后期。进一步的研究表明,用刀豆球蛋白A或氯喹处理完全阻断了AOD诱导的空泡作用,表明该空泡作用取决于酸性溶酶体。总的来说,该结果强烈表明空泡化的增加是由于溶酶体或晚期内体中AOD的积累,从而干扰了溶酶体过程的后期。











































 京公网安备 11010802027423号
京公网安备 11010802027423号