当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetylene Group, Friend or Foe in Medicinal Chemistry.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-02-07 , DOI: 10.1021/acs.jmedchem.9b01617 Tanaji T Talele 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-02-07 , DOI: 10.1021/acs.jmedchem.9b01617 Tanaji T Talele 1
Affiliation
|
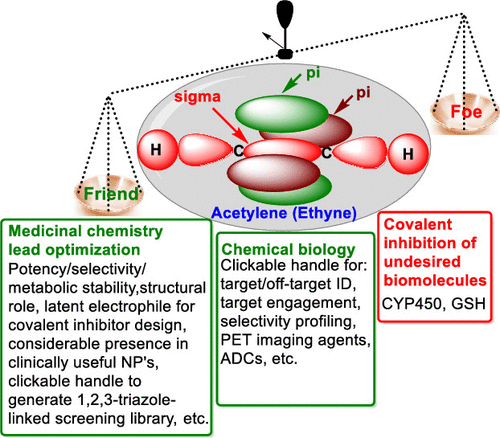
The use of an acetylene (ethynyl) group in medicinal chemistry coincides with the launch of the Journal of Medicinal Chemistry in 1959. Since then, the acetylene group has been broadly exploited in drug discovery and development. As a result, it has become recognized as a privileged structural feature for targeting a wide range of therapeutic target proteins, including MAO, tyrosine kinases, BACE1, steroid receptors, mGlu5 receptors, FFA1/GPR40, and HIV-1 RT. Furthermore, a terminal alkyne functionality is frequently introduced in chemical biology probes as a click handle to identify molecular targets and to assess target engagement. This Perspective is divided into three parts encompassing: (1) the physicochemical properties of the ethynyl group, (2) the advantages and disadvantages of the ethynyl group in medicinal chemistry, and (3) the impact of the ethynyl group on chemical biology approaches.
中文翻译:

药物化学中的乙炔基,敌友。
乙炔基(乙炔基)在药物化学中的使用与《药物化学杂志》的发布同时进行1959年。从那时起,乙炔基已在药物发现和开发中得到广泛利用。结果,它已被公认为是靶向广泛治疗靶蛋白的特权结构特征,包括MAO,酪氨酸激酶,BACE1,类固醇受体,mGlu5受体,FFA1 / GPR40和HIV-1 RT。此外,在化学生物学探针中经常引入末端炔烃功能作为点击手柄,以识别分子靶标并评估靶标参与。该观点分为三个部分,包括:(1)乙炔基的物理化学性质,(2)乙炔基在药物化学中的优缺点,以及(3)乙炔基对化学生物学方法的影响。
更新日期:2020-02-07
中文翻译:

药物化学中的乙炔基,敌友。
乙炔基(乙炔基)在药物化学中的使用与《药物化学杂志》的发布同时进行1959年。从那时起,乙炔基已在药物发现和开发中得到广泛利用。结果,它已被公认为是靶向广泛治疗靶蛋白的特权结构特征,包括MAO,酪氨酸激酶,BACE1,类固醇受体,mGlu5受体,FFA1 / GPR40和HIV-1 RT。此外,在化学生物学探针中经常引入末端炔烃功能作为点击手柄,以识别分子靶标并评估靶标参与。该观点分为三个部分,包括:(1)乙炔基的物理化学性质,(2)乙炔基在药物化学中的优缺点,以及(3)乙炔基对化学生物学方法的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号