当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular-Level Mechanism of Phosphoric Acid Digestion of Carbonates and Recalibration of the 13C–18O Clumped Isotope Thermometer
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-02-24 , DOI: 10.1021/acsearthspacechem.9b00307 Siting Zhang 1, 2, 3 , Qi Liu 2 , Mao Tang 2 , Yun Liu 2, 3
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-02-24 , DOI: 10.1021/acsearthspacechem.9b00307 Siting Zhang 1, 2, 3 , Qi Liu 2 , Mao Tang 2 , Yun Liu 2, 3
Affiliation
|
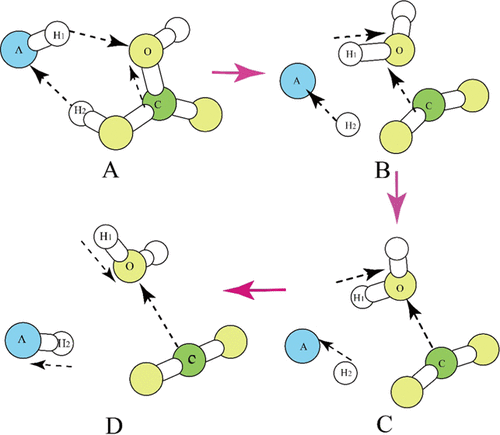
The kinetic isotope effect (KIE) produced by the phosphoric acid digestion reaction of carbonates has been well studied since the beginning of stable isotope geochemistry, but its molecular-level mechanism remains elusive. Importantly, the validity of carbonate 13C–18O clumped isotope thermometry, which is heavily based on the phosphoric acid digestion treatment, needs further study. The existing Δ47–T relationships calibrated by different groups are incompatible and create substantial confusion in the community. Here, we propose a new model of the molecular-level mechanism of the phosphoric acid digestion reaction of carbonates. This new model, which points out that there are three parallel pathways undergoing for the phosphoric acid digestion process, can explain a large part of discrepancies of the Δ47–T relationships obtained by different groups in slightly different experimental conditions. Specifically, it provides different clumped isotope enrichment factors for the reaction at 25, 70, and 90 °C than previous theoretical studies. Under the absolute reference frame (ARF) treatment, these factors are important to obtain comparable Δ47–T relationships. Combined with the equilibrium clumped isotope fractionation factors, which are recalculated using higher-order theoretical treatments, e.g., the anharmonic corrections, a new theoretical Δ47–T relationship is built. This new relationship is no longer a single line but a range that varies to a small extent due to the changes of individual contributions of the three parallel pathways and reflects slight differences in experimental procedures and conditions. These calibration lines have a constant slope close to those of digested at 90 °C but significantly smaller than those at 25 °C. Based on linear mathematical analysis, an unknown isotope effect causing heavy O isotope enrichment during the experiments at 25 °C can be clearly identified. We speculate that this hidden factor was the cause of the rise of slopes. Using a strictly controlled experiment, the distribution of those calibration lines at 90 °C can be largely narrowed down, providing a good base for constructing an ideal clumped isotope thermometer. Extraction of CO2 from carbonate by phosphoric acid digestion and measurement of 17O in CO2 have been used to obtain accurate 17O isotope compositions in carbonate minerals. Our three-pathway mechanism model also provides good predictions of triple oxygen isotope relationships during the phosphoric acid digestion process.
中文翻译:

碳酸盐磷酸消化的分子水平机理和13 C- 18 O丛集同位素温度计的重新校准
自稳定同位素地球化学开始以来,就已经对碳酸盐的磷酸消解反应产生的动力学同位素效应(KIE)进行了充分的研究,但其分子水平的机理仍然难以捉摸。重要的是,很大程度上依赖于磷酸消解处理的碳酸盐13 C- 18 O团簇同位素测温法的有效性需要进一步研究。现有的Δ 47 - Ť由不同群体校准的关系是不相容的,并在社区中造成很大的混乱。在这里,我们提出了碳酸盐磷酸消化反应的分子水平机理的新模型。这种新的模型,它指出有正在进行的磷酸消化过程的三个平行的途径,可以解释Δ的差异很大一部分47 - Ť在略微不同的实验条件下通过不同的基团而获得的关系。具体而言,与之前的理论研究相比,它为25、70和90°C下的反应提供了不同的聚集同位素富集因子。下的绝对参考帧(ARF)的治疗,这些因素是重要的,以获得比较的Δ 47 - Ť关系。结合平衡结块同位素分馏因子,其是使用高阶理论处理,例如,非谐校正,一个新的理论Δ重新计算47 - Ť关系建立。这种新的关系不再是一条直线,而是由于三个平行途径的个体贡献的变化而在很小范围内变化的范围,并且反映了实验程序和条件的细微差异。这些校准线的恒定斜率接近于90°C消化的斜率,但远小于25°C消化的斜率。根据线性数学分析,可以清楚地识别出在25°C的实验过程中引起大量O同位素富集的未知同位素效应。我们推测此隐藏因素是坡度上升的原因。通过严格控制的实验,可以大大缩小90°C下那些校准线的分布范围,从而为构建理想的成簇同位素温度计提供了良好的基础。一氧化碳的萃取2从由磷酸消化和测量碳酸盐17中COÒ 2已被用来获得精确的17在碳酸盐矿物氧同位素的组合物。我们的三途径机理模型还提供了磷酸消化过程中三重氧同位素关系的良好预测。
更新日期:2020-02-24
中文翻译:

碳酸盐磷酸消化的分子水平机理和13 C- 18 O丛集同位素温度计的重新校准
自稳定同位素地球化学开始以来,就已经对碳酸盐的磷酸消解反应产生的动力学同位素效应(KIE)进行了充分的研究,但其分子水平的机理仍然难以捉摸。重要的是,很大程度上依赖于磷酸消解处理的碳酸盐13 C- 18 O团簇同位素测温法的有效性需要进一步研究。现有的Δ 47 - Ť由不同群体校准的关系是不相容的,并在社区中造成很大的混乱。在这里,我们提出了碳酸盐磷酸消化反应的分子水平机理的新模型。这种新的模型,它指出有正在进行的磷酸消化过程的三个平行的途径,可以解释Δ的差异很大一部分47 - Ť在略微不同的实验条件下通过不同的基团而获得的关系。具体而言,与之前的理论研究相比,它为25、70和90°C下的反应提供了不同的聚集同位素富集因子。下的绝对参考帧(ARF)的治疗,这些因素是重要的,以获得比较的Δ 47 - Ť关系。结合平衡结块同位素分馏因子,其是使用高阶理论处理,例如,非谐校正,一个新的理论Δ重新计算47 - Ť关系建立。这种新的关系不再是一条直线,而是由于三个平行途径的个体贡献的变化而在很小范围内变化的范围,并且反映了实验程序和条件的细微差异。这些校准线的恒定斜率接近于90°C消化的斜率,但远小于25°C消化的斜率。根据线性数学分析,可以清楚地识别出在25°C的实验过程中引起大量O同位素富集的未知同位素效应。我们推测此隐藏因素是坡度上升的原因。通过严格控制的实验,可以大大缩小90°C下那些校准线的分布范围,从而为构建理想的成簇同位素温度计提供了良好的基础。一氧化碳的萃取2从由磷酸消化和测量碳酸盐17中COÒ 2已被用来获得精确的17在碳酸盐矿物氧同位素的组合物。我们的三途径机理模型还提供了磷酸消化过程中三重氧同位素关系的良好预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号