当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Postfunctionalization of a Perfluoroarene-Containing π-Conjugated Polymer via Nucleophilic Aromatic Substitution Reaction
ACS Macro Letters ( IF 5.1 ) Pub Date : 2020-02-07 , DOI: 10.1021/acsmacrolett.9b01020 Kazuyuki Ninomiya 1 , Naoki Shida 1 , Takanobu Nishikawa 1 , Takuya Ishihara 1 , Hiroki Nishiyama 1 , Ikuyoshi Tomita 1 , Shinsuke Inagi 1, 2
ACS Macro Letters ( IF 5.1 ) Pub Date : 2020-02-07 , DOI: 10.1021/acsmacrolett.9b01020 Kazuyuki Ninomiya 1 , Naoki Shida 1 , Takanobu Nishikawa 1 , Takuya Ishihara 1 , Hiroki Nishiyama 1 , Ikuyoshi Tomita 1 , Shinsuke Inagi 1, 2
Affiliation
|
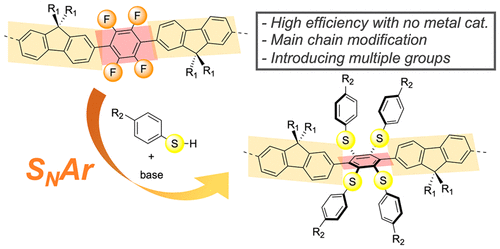
Postfunctionalization is a useful strategy to tune the properties of conjugated polymers, while polymer reactions in the main chain of a conjugated backbone are still underexplored. Here we report the postfunctionalization of the main chain of a conjugated polymer via nucleophilic aromatic substitution reaction. Poly(9,9-dioctylfluorene-alt-tetrafluoro-p-phenylene) is used as a precursor to react with thiophenol derivatives in the presence of a base to enable multiple introduction of arylthio groups into the polymer main chain in high yield with preserving the backbone and the dispersity of the precursor polymer. The main chain structure and optoelectronic properties of the resulting polymers were significantly changed, evidenced by spectroscopic analysis of both model compounds and polymers as well as a computational simulation.
中文翻译:

通过亲核芳族取代反应对含全氟芳烃的 π-共轭聚合物进行后官能化
后官能化是调整共轭聚合物性质的有用策略,而共轭主链主链中的聚合物反应仍未得到充分探索。在这里,我们报告了通过亲核芳族取代反应对共轭聚合物主链进行后官能化。聚(9,9-二辛基芴-alt-四氟-p-亚苯基)用作前体,在碱存在下与苯硫酚衍生物反应,从而能够以高产率将芳硫基多次引入聚合物主链,同时保持前体聚合物的主链和分散性。所得聚合物的主链结构和光电特性发生了显着变化,模型化合物和聚合物的光谱分析以及计算模拟证明了这一点。
更新日期:2020-02-07
中文翻译:

通过亲核芳族取代反应对含全氟芳烃的 π-共轭聚合物进行后官能化
后官能化是调整共轭聚合物性质的有用策略,而共轭主链主链中的聚合物反应仍未得到充分探索。在这里,我们报告了通过亲核芳族取代反应对共轭聚合物主链进行后官能化。聚(9,9-二辛基芴-alt-四氟-p-亚苯基)用作前体,在碱存在下与苯硫酚衍生物反应,从而能够以高产率将芳硫基多次引入聚合物主链,同时保持前体聚合物的主链和分散性。所得聚合物的主链结构和光电特性发生了显着变化,模型化合物和聚合物的光谱分析以及计算模拟证明了这一点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号