Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
YAP Regulates Hematopoietic Stem Cell Formation in Response to the Biomechanical Forces of Blood Flow.
Developmental Cell ( IF 10.7 ) Pub Date : 2020-02-06 , DOI: 10.1016/j.devcel.2020.01.006 Vanessa Lundin 1 , Wade W Sugden 2 , Lindsay N Theodore 2 , Patricia M Sousa 3 , Areum Han 3 , Stephanie Chou 3 , Paul J Wrighton 4 , Andrew G Cox 4 , Donald E Ingber 5 , Wolfram Goessling 6 , George Q Daley 7 , Trista E North 2
Developmental Cell ( IF 10.7 ) Pub Date : 2020-02-06 , DOI: 10.1016/j.devcel.2020.01.006 Vanessa Lundin 1 , Wade W Sugden 2 , Lindsay N Theodore 2 , Patricia M Sousa 3 , Areum Han 3 , Stephanie Chou 3 , Paul J Wrighton 4 , Andrew G Cox 4 , Donald E Ingber 5 , Wolfram Goessling 6 , George Q Daley 7 , Trista E North 2
Affiliation
|
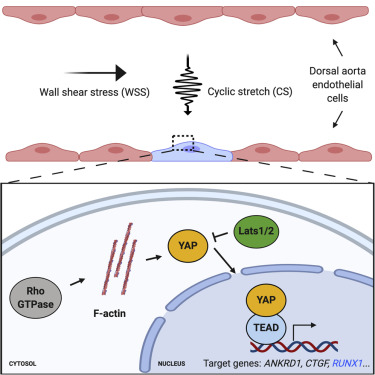
Hematopoietic stem and progenitor cells (HSPCs), first specified from hemogenic endothelium (HE) in the ventral dorsal aorta (VDA), support lifelong hematopoiesis. Their de novo production promises significant therapeutic value; however, current in vitro approaches cannot efficiently generate multipotent long-lived HSPCs. Presuming this reflects a lack of extrinsic cues normally impacting the VDA, we devised a human dorsal aorta-on-a-chip platform that identified Yes-activated protein (YAP) as a cyclic stretch-induced regulator of HSPC formation. In the zebrafish VDA, inducible Yap overexpression significantly increased runx1 expression in vivo and the number of CD41+ HSPCs downstream of HE specification. Endogenous Yap activation by lats1/2 knockdown or Rho-GTPase stimulation mimicked Yap overexpression and induced HSPCs in embryos lacking blood flow. Notably, in static human induced pluripotent stem cell (iPSC)-derived HE culture, compound-mediated YAP activation enhanced RUNX1 levels and hematopoietic colony-forming potential. Together, our findings reveal a potent impact of hemodynamic Rho-YAP mechanotransduction on HE fate, relevant to de novo human HSPC production.
中文翻译:

YAP 响应血流的生物力学力调节造血干细胞的形成。
造血干细胞和祖细胞 (HSPCs) 最初由腹背主动脉 (VDA) 中的造血内皮 (HE) 指定,支持终生造血。它们的从头生产有望带来显着的治疗价值;然而,目前的体外方法不能有效地产生多能长寿命 HSPC。假设这反映了缺乏通常影响 VDA 的外在线索,我们设计了一个人类背主动脉芯片平台,该平台将 Yes 激活蛋白 (YAP) 确定为循环拉伸诱导的 HSPC 形成调节剂。在斑马鱼 VDA 中,诱导型 Yap 过表达显着增加体内 runx1 表达和 HE 规范下游 CD41+ HSPC 的数量。通过 lats1/2 敲低或 Rho-GTPase 刺激激活内源性 Yap 模拟了 Yap 过表达并在缺乏血流的胚胎中诱导 HSPC。值得注意的是,在静态人类诱导多能干细胞 (iPSC) 衍生的 HE 培养物中,化合物介导的 YAP 激活增强了 RUNX1 水平和造血集落形成潜力。总之,我们的研究结果揭示了血流动力学 Rho-YAP 机械转导对 HE 命运的强大影响,与从头人类 HSPC 生产相关。
更新日期:2020-02-07
中文翻译:

YAP 响应血流的生物力学力调节造血干细胞的形成。
造血干细胞和祖细胞 (HSPCs) 最初由腹背主动脉 (VDA) 中的造血内皮 (HE) 指定,支持终生造血。它们的从头生产有望带来显着的治疗价值;然而,目前的体外方法不能有效地产生多能长寿命 HSPC。假设这反映了缺乏通常影响 VDA 的外在线索,我们设计了一个人类背主动脉芯片平台,该平台将 Yes 激活蛋白 (YAP) 确定为循环拉伸诱导的 HSPC 形成调节剂。在斑马鱼 VDA 中,诱导型 Yap 过表达显着增加体内 runx1 表达和 HE 规范下游 CD41+ HSPC 的数量。通过 lats1/2 敲低或 Rho-GTPase 刺激激活内源性 Yap 模拟了 Yap 过表达并在缺乏血流的胚胎中诱导 HSPC。值得注意的是,在静态人类诱导多能干细胞 (iPSC) 衍生的 HE 培养物中,化合物介导的 YAP 激活增强了 RUNX1 水平和造血集落形成潜力。总之,我们的研究结果揭示了血流动力学 Rho-YAP 机械转导对 HE 命运的强大影响,与从头人类 HSPC 生产相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号