Journal of Autoimmunity ( IF 7.9 ) Pub Date : 2020-02-06 , DOI: 10.1016/j.jaut.2020.102417 Joseph W Dean 1 , Leeana D Peters 2 , Christopher A Fuhrman 3 , Howard R Seay 4 , Amanda L Posgai 2 , Scott E Stimpson 2 , Maigan A Brusko 2 , Daniel J Perry 2 , Wen-I Yeh 4 , Brittney N Newby 5 , Michael J Haller 6 , Andrew B Muir 7 , Mark A Atkinson 8 , Clayton E Mathews 9 , Todd M Brusko 9
|
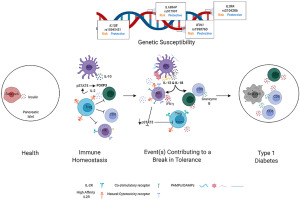
IL-12 and IL-18 synergize to promote TH1 responses and have been implicated as accelerators of autoimmune pathogenesis in type 1 diabetes (T1D). We investigated the influence of these cytokines on immune cells involved in human T1D progression: natural killer (NK) cells, regulatory T cells (Tregs), and cytotoxic T lymphocytes (CTL). NK cells from T1D patients exhibited higher surface CD226 versus controls and lower CD25 compared to first-degree relatives and controls. Changes in NK cell phenotype towards terminal differentiation were associated with cytomegalovirus (CMV) seropositivity, while possession of IL18RAP, IFIH1, and IL2RA T1D-risk variants impacted NK cell activation as evaluated by immuno-expression quantitative trait loci (eQTL) analyses. IL-12 and IL-18 stimulated NK cells from healthy donors exhibited enhanced specific killing of myelogenous K562 target cells. Moreover, activated NK cells increased expression of NKG2A, NKG2D, CD226, TIGIT and CD25, which enabled competition for IL-2 upon co-culture with Tregs, resulting in Treg downregulation of FOXP3, production of IFNγ, and loss of suppressive function. We generated islet-autoreactive CTL “avatars”, which upon exposure to IL-12 and IL-18, upregulated IFNγ and Granzyme-B leading to increased lymphocytotoxicity of a human β-cell line in vitro. These results support a model for T1D pathogenesis wherein IL-12 and IL-18 synergistically enhance CTL and NK cell cytotoxic activity and disrupt immunoregulation by Tregs.
中文翻译:

先天性炎症会驱动 NK 细胞激活,从而损害 Treg 活性。
IL-12 和 IL-18 协同促进 T H 1 反应,并被认为是 1 型糖尿病 (T1D) 自身免疫发病机制的加速器。我们研究了这些细胞因子对参与人类 T1D 进展的免疫细胞的影响:自然杀伤 (NK) 细胞、调节性 T 细胞 (Treg) 和细胞毒性 T 淋巴细胞 (CTL)。来自 T1D 患者的 NK 细胞与对照组相比表现出更高的表面 CD226,与一级亲属和对照组相比表现出更低的 CD25。 NK 细胞表型向终末分化的变化与巨细胞病毒 (CMV) 血清阳性相关,而通过免疫表达数量性状位点 (eQTL) 分析评估, IL18RAP 、 IFIH1和IL2RA T1D 风险变异会影响 NK 细胞活化。来自健康供体的 IL-12 和 IL-18 刺激的 NK 细胞表现出对骨髓 K562 靶细胞的特异性杀伤增强。此外,活化的NK细胞增加了NKG2A、NKG2D、CD226、TIGIT和CD25的表达,从而在与Tregs共培养时竞争IL-2,导致Treg细胞下调FOXP3、产生IFNγ并丧失抑制功能。我们生成了胰岛自身反应性 CTL“化身”,其在暴露于 IL-12 和 IL-18 后,上调 IFNγ 和粒酶-B,导致体外人 β 细胞系的淋巴细胞毒性增加。这些结果支持 T1D 发病机制的模型,其中 IL-12 和 IL-18 协同增强 CTL 和 NK 细胞的细胞毒活性并破坏 Tregs 的免疫调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号