当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.accounts.9b00569 Jingchao Li 1 , Kanyi Pu 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.accounts.9b00569 Jingchao Li 1 , Kanyi Pu 1
Affiliation
|
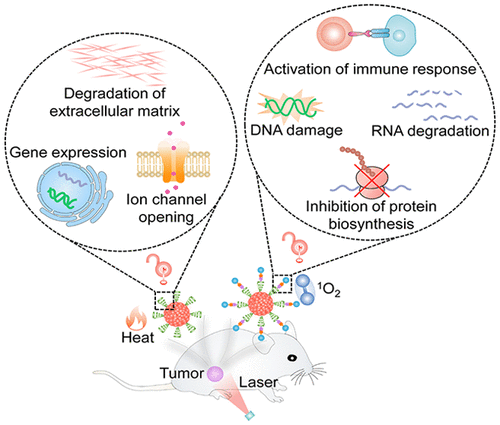
ConspectusCancer therapy is routinely performed in the clinic to cure cancer and control its progression, wherein therapeutic agents are generally used. To reduce side effects, protherapeutic agents that can be activated by overexpressed cancer biomarkers are under development. However, these agents still face certain extent of off-target activation in normal tissues, stimulating the interest to design external-stimuli activatable protherapeutics. In this regard, photoactivatable protherapeutic agents have been utilized for cancer treatments. However, because of the intrinsic features of photolabile moieties, most photoactivatable protherapeutic agents only respond to ultraviolet-visible light, limiting their in vivo applications. Thus, protherapeutic agents that can be activated by near-infrared (NIR) light with minimal phototoxicity and increased tissue penetration are highly desired.In this Account, we summarize our semiconducting polymer nanomaterials (SPNs) as NIR photoactivatable protherapeutic agents for cancer treatment. SPNs are transformed from π-conjugated polymers that efficiently convert NIR light into heat or singlet oxygen (1O2). With photothermal and photodynamic properties, SPNs can be directly used as photomedicine or serve as light transducers to activate heat or 1O2-responsive protherapeutic agents.The heat-activatable SPN-based protherapeutic agents are developed by loading or conjugating of SPNs with therapeutic agents (e.g., agonist, gene, and enzyme). For instance, photothermally triggered release of agonists specifically activates certain protein ion channels on the cellular membrane, leading to ion overinflux induced mitochondria dysfunction and consequently apoptosis of cancer cells. Moreover, photothermal activation of temperature-sensitive bromelain can promote the in situ degradation of collagens (the major components of extracellular matrix), resulting in an improved accumulation of agents in tumor tissues and thus amplified therapeutic outcome.The 1O2-activatable SPN-based protherapeutic agents are constructed through covalent conjugation of SPNs with caged therapeutic agents via hypoxia- or 1O2-cleavable linkers. Upon NIR photoirradiation, SPNs consume oxygen to generate 1O2, which leads to photodynamic therapy (PDT), and meanwhile breaks hypoxia- or 1O2-cleavable linkers for on-demand release and in situ activation of caged protherapeutic molecules (e.g., chemodrug, enzyme, and inhibitor). Such remote activation of SPN-based protherapeutic agents can be applied to induce DNA damage, ribonucleic acid degradation, inhibition of protein biosynthesis, or immune system activation in tumors of living animals. By synergizing PDT with NIR photoactivation of those biological actions, these protherapeutic agents effectively eliminate tumors and even fully inhibit tumor metastasis.This Account highlights the potential of SPNs for construction of versatile NIR photoactivatable protherapeutics to treat cancer at designated times and locations with high therapeutic outcome and precision.
中文翻译:

半导体聚合物纳米材料作为癌症的近红外光活化疗法。
ConspectusCancer疗法通常在临床上进行以治愈癌症并控制其进展,其中通常使用治疗剂。为了减少副作用,可以被过表达的癌症生物标志物激活的治疗药物正在开发中。然而,这些试剂在正常组织中仍然面临一定程度的脱靶激活,激发了人们对设计外部刺激可激活治疗剂的兴趣。在这方面,可光活化的治疗剂已用于癌症治疗。然而,由于光不稳定部分的固有特征,大多数可光活化的治疗剂仅对紫外线-可见光有反应,从而限制了它们在体内的应用。从而,迫切需要能够通过近红外(NIR)光激活并具有最小的光毒性和增加的组织穿透性的前体治疗剂。在此帐户中,我们总结了半导体聚合物纳米材料(SPN)作为可用于癌症治疗的NIR光敏性前体治疗剂。SPN由π共轭聚合物转化而成,该聚合物可将NIR光有效地转化为热量或单线态氧(1O2)。SPN具有光热和光动力特性,可以直接用作光医学或用作光敏剂,以激活热或1O2响应性治疗剂。通过将SPN与治疗剂(例如,负载或缀合)一起开发可热激活的基于SPN的治疗剂。 ,激动剂,基因和酶)。例如,激动剂的光热触发释放会特异性激活细胞膜上的某些蛋白质离子通道,从而导致离子过度流入引起线粒体功能障碍,进而导致癌细胞凋亡。此外,对温度敏感的菠萝蛋白酶的光热激活可以促进胶原蛋白的原位降解(细胞外基质的主要成分),从而改善了肿瘤组织中药剂的积累,从而扩大了治疗效果.1O2可激活的SPN基治疗通过将SPN与笼状治疗剂通过低氧或1O2可裂解的连接子共价缀合来构建这些药物。在近红外光照射下,SPN消耗氧气产生1O2,从而导致光动力疗法(PDT),同时打破了缺氧或1O2可裂解的接头,以按需释放和原位激活笼状治疗分子(例如化学药物,酶和抑制剂)。这种基于SPN的治疗药物的远程激活可用于在活体动物的肿瘤中诱导DNA损伤,核糖核酸降解,抑制蛋白质生物合成或激活免疫系统。通过使PDT与这些生物作用的NIR光活化协同作用,这些治疗药物可有效消除肿瘤,甚至完全抑制肿瘤转移。和精度。和抑制剂)。这种基于SPN的治疗药物的远程活化可用于在活体动物的肿瘤中诱导DNA损伤,核糖核酸降解,抑制蛋白质生物合成或激活免疫系统。通过使PDT与这些生物作用的NIR光活化协同作用,这些治疗药物可以有效消除肿瘤,甚至完全抑制肿瘤转移。该研究着重说明了SPN在构建通用的NIR光活化药物治疗潜在的特定时间和地点具有巨大治疗潜力的潜力。和精度。和抑制剂)。这种基于SPN的治疗药物的远程活化可用于在活体动物的肿瘤中诱导DNA损伤,核糖核酸降解,抑制蛋白质生物合成或激活免疫系统。通过使PDT与这些生物作用的NIR光活化协同作用,这些治疗药物可以有效消除肿瘤,甚至完全抑制肿瘤转移。该研究着重说明了SPN在构建通用的NIR光活化药物治疗潜在的特定时间和地点具有巨大治疗潜力的潜力。和精度。
更新日期:2020-02-06
中文翻译:

半导体聚合物纳米材料作为癌症的近红外光活化疗法。
ConspectusCancer疗法通常在临床上进行以治愈癌症并控制其进展,其中通常使用治疗剂。为了减少副作用,可以被过表达的癌症生物标志物激活的治疗药物正在开发中。然而,这些试剂在正常组织中仍然面临一定程度的脱靶激活,激发了人们对设计外部刺激可激活治疗剂的兴趣。在这方面,可光活化的治疗剂已用于癌症治疗。然而,由于光不稳定部分的固有特征,大多数可光活化的治疗剂仅对紫外线-可见光有反应,从而限制了它们在体内的应用。从而,迫切需要能够通过近红外(NIR)光激活并具有最小的光毒性和增加的组织穿透性的前体治疗剂。在此帐户中,我们总结了半导体聚合物纳米材料(SPN)作为可用于癌症治疗的NIR光敏性前体治疗剂。SPN由π共轭聚合物转化而成,该聚合物可将NIR光有效地转化为热量或单线态氧(1O2)。SPN具有光热和光动力特性,可以直接用作光医学或用作光敏剂,以激活热或1O2响应性治疗剂。通过将SPN与治疗剂(例如,负载或缀合)一起开发可热激活的基于SPN的治疗剂。 ,激动剂,基因和酶)。例如,激动剂的光热触发释放会特异性激活细胞膜上的某些蛋白质离子通道,从而导致离子过度流入引起线粒体功能障碍,进而导致癌细胞凋亡。此外,对温度敏感的菠萝蛋白酶的光热激活可以促进胶原蛋白的原位降解(细胞外基质的主要成分),从而改善了肿瘤组织中药剂的积累,从而扩大了治疗效果.1O2可激活的SPN基治疗通过将SPN与笼状治疗剂通过低氧或1O2可裂解的连接子共价缀合来构建这些药物。在近红外光照射下,SPN消耗氧气产生1O2,从而导致光动力疗法(PDT),同时打破了缺氧或1O2可裂解的接头,以按需释放和原位激活笼状治疗分子(例如化学药物,酶和抑制剂)。这种基于SPN的治疗药物的远程激活可用于在活体动物的肿瘤中诱导DNA损伤,核糖核酸降解,抑制蛋白质生物合成或激活免疫系统。通过使PDT与这些生物作用的NIR光活化协同作用,这些治疗药物可有效消除肿瘤,甚至完全抑制肿瘤转移。和精度。和抑制剂)。这种基于SPN的治疗药物的远程活化可用于在活体动物的肿瘤中诱导DNA损伤,核糖核酸降解,抑制蛋白质生物合成或激活免疫系统。通过使PDT与这些生物作用的NIR光活化协同作用,这些治疗药物可以有效消除肿瘤,甚至完全抑制肿瘤转移。该研究着重说明了SPN在构建通用的NIR光活化药物治疗潜在的特定时间和地点具有巨大治疗潜力的潜力。和精度。和抑制剂)。这种基于SPN的治疗药物的远程活化可用于在活体动物的肿瘤中诱导DNA损伤,核糖核酸降解,抑制蛋白质生物合成或激活免疫系统。通过使PDT与这些生物作用的NIR光活化协同作用,这些治疗药物可以有效消除肿瘤,甚至完全抑制肿瘤转移。该研究着重说明了SPN在构建通用的NIR光活化药物治疗潜在的特定时间和地点具有巨大治疗潜力的潜力。和精度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号