当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Stabilization and Dissolution Rate Improvement of Amorphous Compacts with Thin Polymer Coatings: Can We Have It All?
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.molpharmaceut.9b01263 Dunja Novakovic 1 , Leena Peltonen 1 , Antti Isomäki 2 , Sara J Fraser-Miller 3 , Line Hagner Nielsen 4 , Timo Laaksonen 5 , Clare J Strachan 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.molpharmaceut.9b01263 Dunja Novakovic 1 , Leena Peltonen 1 , Antti Isomäki 2 , Sara J Fraser-Miller 3 , Line Hagner Nielsen 4 , Timo Laaksonen 5 , Clare J Strachan 1
Affiliation
|
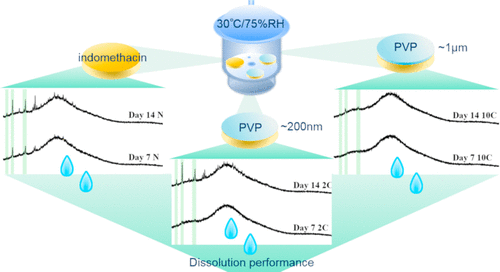
The distinction between surface and bulk crystallization of amorphous pharmaceuticals, as well as the importance of surface crystallization for pharmaceutical performance, is becoming increasingly evident. An emerging strategy in stabilizing the amorphous drug form is to utilize thin coatings at the surface. While the physical stability of systems coated with pharmaceutical polymers has recently been studied, the effect on dissolution performance as a function of storage time, as a further necessary step toward the success of these formulations, has not been previously studied. Furthermore, the effect of coating thickness has not been elucidated. This study investigated the effect of these polymer-coating parameters on the interplay between amorphous surface crystallization and drug dissolution for the first time. The study utilized simple tablet-like coated dosage forms, comprising a continuous amorphous drug core and thin polymer coating (hundreds of nanometers to a micrometer thick). Monitoring included analysis of both the solid-state of the model drug (with SEM, XRD, and ATR FTIR spectroscopy) and dissolution performance (and associated morphology and solid-state changes) after different storage times. Stabilization of the amorphous form (dependent on the coating thickness) and maintenance of early-stage intrinsic dissolution rates characteristic for the unaged amorphous drug were achieved. However, dissolution in the latter stages was likely inhibited by the presence of a polymer at the surface. Overall, this study introduced a versatile coated system for studying the dissolution of thin-coated amorphous dosage forms suitable for different drugs and coating agents. It demonstrated the importance of multiple factors that need to be taken into consideration when aiming to achieve both physical stability and improved release during the shelf life of amorphous formulations.
中文翻译:

具有薄聚合物涂层的无定形压块的表面稳定性和溶解速率提高:我们能拥有全部吗?
无定形药物的表面和本体结晶之间的区别,以及表面结晶对药物性能的重要性,变得越来越明显。稳定无定形药物形式的一种新兴策略是在表面使用薄涂层。尽管最近已研究了用药物聚合物包衣的体系的物理稳定性,但以前尚未研究过对溶解性能的影响(随储存时间的变化),这是迈向这些制剂成功的又一必要步骤。此外,尚未阐明涂层厚度的影响。这项研究首次研究了这些聚合物涂层参数对无定形表面结晶和药物溶解之间相互作用的影响。该研究利用了简单的片剂状包衣剂型,包括连续的无定形药物核心和薄的聚合物包衣(数百纳米至微米厚)。监测包括在不同存储时间后对模型药物的固态(使用SEM,XRD和ATR FTIR光谱分析)和溶解性能(以及相关的形态和固态变化)的分析。实现了无定形形式的稳定化(取决于涂层厚度),并保持了未老化的无定形药物的早期固有溶出速率。然而,在后期的溶解很可能被表面上聚合物的存在所抑制。总体,这项研究引入了一种多功能的包衣系统,用于研究适用于不同药物和包衣剂的薄衣无定形剂型的溶出度。它证明了在无定形制剂的保质期内要实现物理稳定性和改善释放时,必须考虑多个因素的重要性。
更新日期:2020-02-06
中文翻译:

具有薄聚合物涂层的无定形压块的表面稳定性和溶解速率提高:我们能拥有全部吗?
无定形药物的表面和本体结晶之间的区别,以及表面结晶对药物性能的重要性,变得越来越明显。稳定无定形药物形式的一种新兴策略是在表面使用薄涂层。尽管最近已研究了用药物聚合物包衣的体系的物理稳定性,但以前尚未研究过对溶解性能的影响(随储存时间的变化),这是迈向这些制剂成功的又一必要步骤。此外,尚未阐明涂层厚度的影响。这项研究首次研究了这些聚合物涂层参数对无定形表面结晶和药物溶解之间相互作用的影响。该研究利用了简单的片剂状包衣剂型,包括连续的无定形药物核心和薄的聚合物包衣(数百纳米至微米厚)。监测包括在不同存储时间后对模型药物的固态(使用SEM,XRD和ATR FTIR光谱分析)和溶解性能(以及相关的形态和固态变化)的分析。实现了无定形形式的稳定化(取决于涂层厚度),并保持了未老化的无定形药物的早期固有溶出速率。然而,在后期的溶解很可能被表面上聚合物的存在所抑制。总体,这项研究引入了一种多功能的包衣系统,用于研究适用于不同药物和包衣剂的薄衣无定形剂型的溶出度。它证明了在无定形制剂的保质期内要实现物理稳定性和改善释放时,必须考虑多个因素的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号