The Lancet HIV ( IF 12.8 ) Pub Date : 2020-02-06 , DOI: 10.1016/s2352-3018(19)30406-0 Punnee Pitisuttithum 1 , Sorachai Nitayaphan 2 , Suwat Chariyalertsak 3 , Jaranit Kaewkungwal 1 , Peter Dawson 4 , Jittima Dhitavat 1 , Benjaluck Phonrat 1 , Siriwat Akapirat 2 , Nicos Karasavvas 5 , Lindsay Wieczorek 6 , Victoria Polonis 7 , Michael A Eller 6 , Poonam Pegu 6 , Dohoon Kim 6 , Alexandra Schuetz 8 , Surat Jongrakthaitae 2 , Yingjun Zhou 4 , Faruk Sinangil 9 , Sanjay Phogat 10 , Carlos A Diazgranados 11 , James Tartaglia 11 , Elizabeth Heger 12 , Kirsten Smith 2 , Nelson L Michael 7 , Jean-Louis Excler 13 , Merlin L Robb 6 , Jerome H Kim 14 , Robert J O'Connell 15 , Sandhya Vasan 8 ,
|
Background
The RV144 phase 3 vaccine trial in Thailand demonstrated that ALVAC-HIV (vCP1521) and AIDSVAX B/E administration over 6 months resulted in a 31% efficacy in preventing HIV acquisition. In this trial, we assessed the immunological effect of an additional vaccine boost to the RV144 regimen at varying intervals between the priming vaccine series and the boost.
Methods
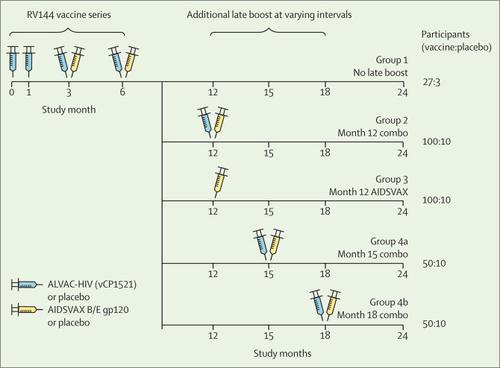
RV306 is a double-blind, placebo-controlled, randomised clinical trial done at three clinical sites in Thailand. Eligible volunteers were HIV-uninfected individuals aged 20–40 years who were at low risk for HIV infection and in good health. A randomisation schedule was centrally generated with fixed sized strata for Research Institute for Health Sciences Chiang Mai and combined Bangkok clinics. Participants were randomly assigned to one of five groups and then further randomly assigned to either vaccine or placebo. All participants received the primary RV144 vaccine series at months 0, 1, 3, and 6. Group 1 received no additional boost, group 2 received additional AIDSVAX B/E and ALVAC-HIV (vCP1521) or placebo at month 12, group 3 received AIDSVAX B/E alone or placebo at month 12, group 4a received AIDSVAX B/E and ALVAC-HIV or placebo at month 15, and group 4b received AIDSVAX B/E and ALVAC-HIV or placebo at month 18. Primary outcomes were safety and tolerability of these vaccination regimens and cellular and humoral immune responses compared between the RV144 series alone and regimens with late boosts at different timepoints. Safety and tolerability outcomes were assessed by evaluating local and systemic reactogenicity and adverse events in all participants. This trial is registered at ClinicalTrials.gov (NCT01931358); clinical follow-up is now complete.
Findings
Between Oct 28, 2013, and April 29, 2014, 367 participants were enrolled, of whom 27 were assigned active vaccination in group 1, 102 in group 2, 101 in group 3, 52 in group 4a, 51 in group 4b, and 34 combined placebo across all the groups. No vaccine-related serious adverse events were recorded. Occurrence and severity of local and systemic reactogenicity were similar across active groups. Groups with late boosts (groups 2, 3, 4a, and 4b) had increased peak plasma IgG-binding antibody levels against gp70 V1V2 relative to group 1 vaccine recipients with no late boost (gp70 V1V2 92TH023 adjusted p<0·02 for each; gp70 V1V2 CaseA2 adjusted p<0·0001 for each). Boosting at month 12 (groups 2 and 3) did not increase gp120 responses compared with the peak responses after the RV144 priming regimen at month 6; however, boosting at month 15 (group 4a) improved responses to gp120 A244gD– D11 (p=0·0003), and boosting at month 18 (group 4b) improved responses to both gp120 A244gD– D11 (p<0·0001) and gp120 MNgD– D11 (p=0·0016). Plasma IgG responses were significantly lower among vaccine recipients boosted at month 12 (pooled groups 2 + 3) than at month 15 (group 4a; adjusted p<0·0001 for each, except for gp70 V1V2 CaseA2, p=0·0142) and at month 18 (group 4b; all adjusted p<0·001). Boosting at month 18 versus month 15 resulted in a significantly higher plasma IgG response to gp120 antigens (all adjusted p<0·01) but not gp70 V1V2 antigens. CD4 functionality and polyfunctionality scores after stimulation with HIV-1 Env peptides (92TH023) increased with delayed boosting. Groups with late boosts had increased functionality and polyfunctionality scores relative to vaccine recipients with no late boost (all adjusted p<0·05, except for the polyfunctionality score in group 1 vs group 4b, p<0·01).
Interpretation
Taken together, these results suggest that additional boosting of the RV144 regimen with longer intervals between the primary vaccination series and late boost improved immune responses and might improve the efficacy of preventing HIV acquisition.
Funding
US National Institute of Allergy and Infectious Diseases and US Department of the Army.
中文翻译:

在未感染 HIV 的泰国志愿者中使用 AIDSVAX B/E 和 ALVAC-HIV 后期加强 RV144 方案:一项双盲、随机对照试验。
背景
在泰国进行的 RV144 3 期疫苗试验表明,服用 ALVAC-HIV (vCP1521) 和 AIDSVAX B/E 超过 6 个月,预防 HIV 感染的功效达到 31%。在这项试验中,我们评估了在初免疫苗系列和加强疫苗之间的不同间隔对 RV144 方案进行额外疫苗加强的免疫学效果。
方法
RV306 是一项在泰国三个临床中心进行的双盲、安慰剂对照、随机临床试验。符合条件的志愿者是年龄在 20 至 40 岁之间、未感染 HIV、HIV 感染风险较低且健康状况良好的个人。随机化时间表是由清迈健康科学研究所和曼谷联合诊所的固定大小分层集中生成的。参与者被随机分配到五组中的一组,然后进一步随机分配到疫苗组或安慰剂组。所有参与者均在第 0、1、3 和 6 个月时接受了 RV144 主要疫苗系列。第 1 组未接受额外加强疫苗接种,第 2 组在第 12 个月接受了额外的 AIDSVAX B/E 和 ALVAC-HIV (vCP1521) 或安慰剂,第 3 组接受了额外疫苗接种第 12 个月时单独使用 AIDSVAX B/E 或安慰剂,第 4a 组在第 15 个月时接受 AIDSVAX B/E 和 ALVAC-HIV 或安慰剂,第 4b 组在第 18 个月时接受 AIDSVAX B/E 和 ALVAC-HIV 或安慰剂。主要结局是安全性以及这些疫苗接种方案的耐受性以及单独 RV144 系列与不同时间点后期加强方案之间的细胞和体液免疫反应的比较。通过评估所有参与者的局部和全身反应原性以及不良事件来评估安全性和耐受性结果。该试验已在 ClinicalTrials.gov 上注册 (NCT01931358);临床随访现已完成。
发现
2013年10月28日至2014年4月29日期间,共有367名参与者入组,其中第1组27人,第2组102人,第3组101人,第4a组52人,第4b组51人,34人所有组均使用联合安慰剂。没有记录到与疫苗相关的严重不良事件。各活动组局部和全身反应原性的发生率和严重程度相似。与没有后期加强的第 1 组疫苗接受者相比,后期加强的组(第 2、3、4a 和 4b 组)针对 gp70 V1V2 的血浆 IgG 结合抗体峰值水平有所增加(gp70 V1V2 92TH023 调整了每个组的 p<0·02; gp70 V1V2 CaseA2 分别调整了 p<0·0001)。与第 6 个月 RV144 初免方案后的峰值反应相比,第 12 个月时的加强(第 2 组和第 3 组)并未增加 gp120 反应;然而,第 15 个月(第 4a 组)的加强治疗改善了对 gp120 A244gD–D11 (p=0·0003) 的反应,第 18 个月(第 4b 组)加强治疗改善了对 gp120 A244gD–D11 (p<0·0001) 和gp120 MNgD–D11 (p=0·0016)。第 12 个月加强接种的疫苗接受者(合并组 2 + 3)中的血浆 IgG 反应显着低于第 15 个月(第 4a 组;每个组均调整后的 p<0·0001,gp70 V1V2 CaseA2 除外,p=0·0142),并且第 18 个月(组 4b;全部调整 p<0·001)。与第 15 个月相比,第 18 个月的加强治疗导致对 gp120 抗原(均经过调整的 p<0·01)但对 gp70 V1V2 抗原的血浆 IgG 应答显着升高。 HIV-1 Env 肽 (92TH023) 刺激后,CD4 功能和多功能性评分随着延迟加强而增加。 与没有后期加强的疫苗接受者相比,后期加强的组的功能性和多功能性得分有所增加(所有调整后的 p<0·05,除了第 1 组与第 4b 组相比的多功能性得分,p<0·01)。
解释
总而言之,这些结果表明,在初次疫苗接种系列和后期加强疫苗接种之间间隔较长的情况下额外加强 RV144 方案可改善免疫反应,并可能提高预防 HIV 感染的功效。
资金
美国国家过敏和传染病研究所和美国陆军部。











































 京公网安备 11010802027423号
京公网安备 11010802027423号