当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a General Automated Flow Photoredox 18F-Difluoromethylation of N-Heteroaromatics in an AllinOne Synthesizer
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.oprd.9b00442 Laura Trump 1, 2 , Agostinho Lemos 1 , Jérôme Jacq 1 , Patrick Pasau 1 , Bénédicte Lallemand 1 , Joël Mercier 1 , Christophe Genicot 1 , André Luxen 2 , Christian Lemaire 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.oprd.9b00442 Laura Trump 1, 2 , Agostinho Lemos 1 , Jérôme Jacq 1 , Patrick Pasau 1 , Bénédicte Lallemand 1 , Joël Mercier 1 , Christophe Genicot 1 , André Luxen 2 , Christian Lemaire 2
Affiliation
|
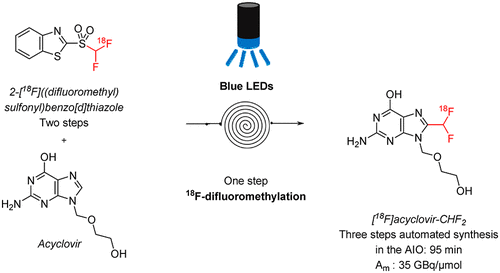
We recently reported a new method for the 18F-difluoromethylation of N-heteroaromatics for positron emission tomography (PET) imaging. The method involves the synthesis of a new 18F-difluoromethylating reagent, 2-[18F]((difluoromethyl)sulfonyl)benzo[d]thiazole, and a flow photoredox 18F-difluoromethylation. For preclinical development and human PET studies with new radiotracers, automation of the process is mandatory, mostly to avoid radioprotection issues due to the use of high amounts of radioactivity and to ensure better reliability of the production. Herein we describe the automation of this 18F-difluoromethylation method on a model substrate, acyclovir, on a commercially available AllinOne (AIO) synthesizer from Trasis. The whole process is completed in 95 min and provides radiolabeled acyclovir with a molar activity of 35 GBq/μmol. This automated protocol can be implemented for the 18F-difluoromethylation of a wide range of N-heteroaromatic compounds typically found in medicinal chemistry.
中文翻译:

在AllinOne合成器中进行N-杂芳族化合物的一般自动化流光氧化还原18 F-二氟甲基化的开发。
我们最近报道了一种用于正电子发射断层扫描(PET)成像的N-杂芳族化合物18 F-二氟甲基化的新方法。该方法包括合成新的18 F-二氟甲基化试剂2- [ 18 F](((二氟甲基)磺酰基)苯并[ d ]噻唑和流动光氧化还原18 F-二氟甲基化。对于临床前开发和使用新的放射性示踪剂的人类PET研究,该过程的自动化是强制性的,主要是为了避免由于使用大量放射性而产生的辐射防护问题,并确保更好的生产可靠性。在这里,我们描述这18个自动化在市售的Trasis的AllinOne(AIO)合成仪上,在模型基质阿昔洛韦上进行F-二氟甲基化方法。整个过程在95分钟内完成,并提供放射性标记的阿昔洛韦,摩尔活性为35 GBq /μmol。可以对通常在药物化学中发现的各种N-杂芳族化合物进行18 F-二氟甲基化来实现此自动化方案。
更新日期:2020-02-06
中文翻译:

在AllinOne合成器中进行N-杂芳族化合物的一般自动化流光氧化还原18 F-二氟甲基化的开发。
我们最近报道了一种用于正电子发射断层扫描(PET)成像的N-杂芳族化合物18 F-二氟甲基化的新方法。该方法包括合成新的18 F-二氟甲基化试剂2- [ 18 F](((二氟甲基)磺酰基)苯并[ d ]噻唑和流动光氧化还原18 F-二氟甲基化。对于临床前开发和使用新的放射性示踪剂的人类PET研究,该过程的自动化是强制性的,主要是为了避免由于使用大量放射性而产生的辐射防护问题,并确保更好的生产可靠性。在这里,我们描述这18个自动化在市售的Trasis的AllinOne(AIO)合成仪上,在模型基质阿昔洛韦上进行F-二氟甲基化方法。整个过程在95分钟内完成,并提供放射性标记的阿昔洛韦,摩尔活性为35 GBq /μmol。可以对通常在药物化学中发现的各种N-杂芳族化合物进行18 F-二氟甲基化来实现此自动化方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号