当前位置:
X-MOL 学术
›
Am. J. Transplant.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: A systematic review.
American Journal of Transplantation ( IF 8.9 ) Pub Date : 2020-02-06 , DOI: 10.1111/ajt.15811 Thibaut d'Izarny-Gargas 1 , Antoine Durrbach 2, 3, 4 , Mohamad Zaidan 1, 3
American Journal of Transplantation ( IF 8.9 ) Pub Date : 2020-02-06 , DOI: 10.1111/ajt.15811 Thibaut d'Izarny-Gargas 1 , Antoine Durrbach 2, 3, 4 , Mohamad Zaidan 1, 3
Affiliation
|
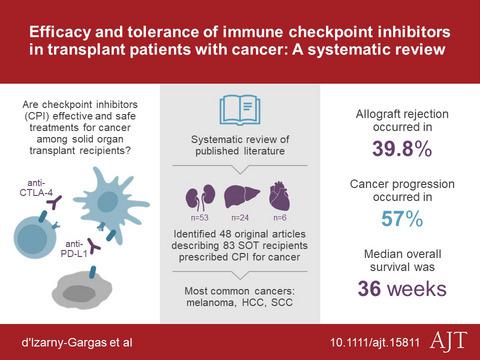
Solid organ transplant (SOT) is frequently complicated by cancers, which render immunosuppression challenging. Immune checkpoint inhibitors have emerged as treatments for many cancers. Data are lacking regarding efficacy and rejection risk in the SOT population. We conducted a systematic literature review and analyzed 83 cases of immune checkpoint inhibitor use for cancer in SOT. Two thirds of these patients received anti–programmed death ligand 1 therapy, 15.7% received anti–cytotoxic T lymphocyte–associated protein 4 therapy, and 10.8% received a combination. Allograft rejection occurred in 39.8% of patients, leading to end‐stage organ failure in 71.0% of cases. Outcomes were similar across organs and immunotherapy regimens. The use of immunosuppressants other than steroids, time since transplant, and prior episodes of rejection were associated with the risk of rejection. The median overall survival of patients was 36 weeks. Most of the deaths were related to cancer progression. In nonkidney recipients, graft rejection was strongly associated with worse survival. At the end of the study, 19.3% of the patients were alive, free from rejection and tumor progression. This study highlights the difficult tradeoff facing oncologists and transplant specialists managing transplant recipients with cancer, and the need for prospective data and novel biomarkers for identifying the patients likely to benefit from immunotherapy in the SOT setting.
中文翻译:

免疫检查点抑制剂在移植癌症患者中的疗效和耐受性:系统评价。
实体器官移植 (SOT) 经常并发癌症,这使得免疫抑制具有挑战性。免疫检查点抑制剂已成为许多癌症的治疗药物。缺乏关于 SOT 人群疗效和排斥风险的数据。我们进行了系统的文献回顾,并分析了 83 例免疫检查点抑制剂在 SOT 中用于癌症的情况。这些患者中有三分之二接受了抗程序性死亡配体 1 治疗,15.7% 接受了抗细胞毒性 T 淋巴细胞相关蛋白 4 治疗,10.8% 接受了联合治疗。39.8% 的患者发生同种异体移植物排斥反应,导致 71.0% 的患者出现终末期器官衰竭。不同器官和免疫治疗方案的结果相似。使用类固醇以外的免疫抑制剂,移植后的时间,之前的排斥事件与排斥风险有关。患者的中位总生存期为 36 周。大多数死亡与癌症进展有关。在非肾脏接受者中,移植物排斥与较差的存活率密切相关。在研究结束时,19.3% 的患者还活着,没有排斥反应和肿瘤进展。这项研究强调了肿瘤学家和移植专家在管理患有癌症的移植受者时面临的艰难权衡,以及需要前瞻性数据和新的生物标志物来识别可能从 SOT 环境中的免疫疗法中受益的患者。移植物排斥与较差的存活率密切相关。在研究结束时,19.3% 的患者还活着,没有排斥反应和肿瘤进展。这项研究强调了肿瘤学家和移植专家在管理患有癌症的移植受者时面临的艰难权衡,以及需要前瞻性数据和新的生物标志物来识别可能从 SOT 环境中的免疫疗法中受益的患者。移植物排斥与较差的存活率密切相关。在研究结束时,19.3% 的患者还活着,没有排斥反应和肿瘤进展。这项研究强调了肿瘤学家和移植专家在管理患有癌症的移植受者时面临的艰难权衡,以及需要前瞻性数据和新的生物标志物来识别可能从 SOT 环境中的免疫疗法中受益的患者。
更新日期:2020-02-06
中文翻译:

免疫检查点抑制剂在移植癌症患者中的疗效和耐受性:系统评价。
实体器官移植 (SOT) 经常并发癌症,这使得免疫抑制具有挑战性。免疫检查点抑制剂已成为许多癌症的治疗药物。缺乏关于 SOT 人群疗效和排斥风险的数据。我们进行了系统的文献回顾,并分析了 83 例免疫检查点抑制剂在 SOT 中用于癌症的情况。这些患者中有三分之二接受了抗程序性死亡配体 1 治疗,15.7% 接受了抗细胞毒性 T 淋巴细胞相关蛋白 4 治疗,10.8% 接受了联合治疗。39.8% 的患者发生同种异体移植物排斥反应,导致 71.0% 的患者出现终末期器官衰竭。不同器官和免疫治疗方案的结果相似。使用类固醇以外的免疫抑制剂,移植后的时间,之前的排斥事件与排斥风险有关。患者的中位总生存期为 36 周。大多数死亡与癌症进展有关。在非肾脏接受者中,移植物排斥与较差的存活率密切相关。在研究结束时,19.3% 的患者还活着,没有排斥反应和肿瘤进展。这项研究强调了肿瘤学家和移植专家在管理患有癌症的移植受者时面临的艰难权衡,以及需要前瞻性数据和新的生物标志物来识别可能从 SOT 环境中的免疫疗法中受益的患者。移植物排斥与较差的存活率密切相关。在研究结束时,19.3% 的患者还活着,没有排斥反应和肿瘤进展。这项研究强调了肿瘤学家和移植专家在管理患有癌症的移植受者时面临的艰难权衡,以及需要前瞻性数据和新的生物标志物来识别可能从 SOT 环境中的免疫疗法中受益的患者。移植物排斥与较差的存活率密切相关。在研究结束时,19.3% 的患者还活着,没有排斥反应和肿瘤进展。这项研究强调了肿瘤学家和移植专家在管理患有癌症的移植受者时面临的艰难权衡,以及需要前瞻性数据和新的生物标志物来识别可能从 SOT 环境中的免疫疗法中受益的患者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号